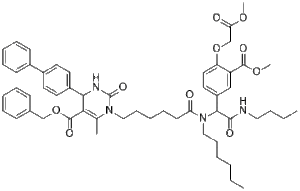
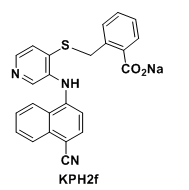
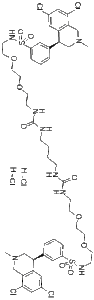
Methyldopate is a novel and potent antihypertensive agent
MAL3-101 is a novel and potent heat shock protein 70 molecular chaperone inhibitor
AVG-233 is a novel and potent blocker of RNA-dependent RNA polymerase (RdRp)
Boc-Lys(Z)-OH is a peptide compound.
Ferutinin is natural product.
KPH2f is a novel, orally bioactive and potent dual URAT1/GLUT9 inhibitor with the potential to be used an anti-hyperuricemic agent for gout treatment.
Tenapanor 2HCl (formerly AZD-1722; AZD1722; RDX-5791; RDX 5791; Ibsrela), the dihydrochloride salt of Tenapanor, is a novel and potent inhibitor of the sodium-proton (Na(+)/H(+)) exchanger NHE3 approved in 2019 for the treatment of irritable bowel syndrome with constipation (IBS-C).
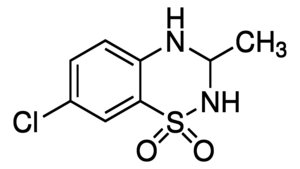
IDRA-21 is a positive allosteric modulator (PAM) of the AMPA receptor and a benzothiadiazine derivative.
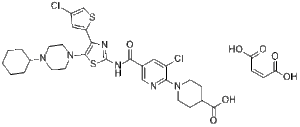
Avatrombopag maleate (formerly AKR501, YM477, AS1670542; E5501; AKR-501, YM-477, AS-1670542; E-5501; Doptelet), the maleate salt of Avatrombopag, is an orally bioactive thrombopoietin (TPO) receptor agonist approved in 2008 to treat patients with chronic idiopathic thrombocytopenic purpura.
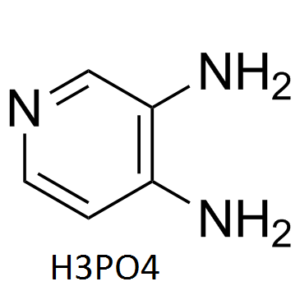
Amifampridine phosphate (trade name: Firdapse; pyridine-3,4-diamine, 3,4-diaminopyridine, 3,4-DAP) is the phosphate salt of Amifampridine, which is an FDA approved drug used mainly in the treatment of a number of rare muscle diseases such as Lambert-Eaton myasthenic syndrome (LEMS) in adults (It gained US approval in November 2018).