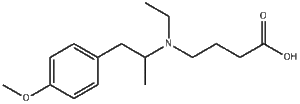
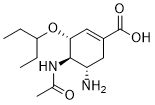
Mebeverine acid is a metabolite of Mebeverine (a musculotropic antispasmodic and anti-irritable bowel syndrome drug).
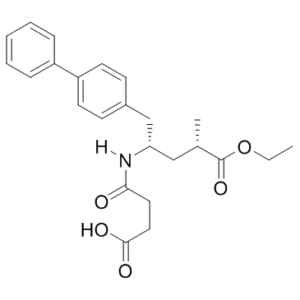
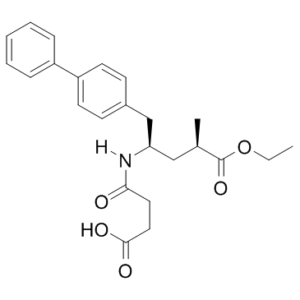
2S,4S-Sacubitril is an impurity of Sacubitril (AHU 377; AHU377; AHU-377), which is a US FDA approved drug used in combination with valsartan (trade name Entrosto for the combo)for the treatment of heart failure.
DCBA (DEET ω-Carboxylic Acid) is a metabolite of insect repellent N-N-diethyl-meta-toluamide (DEET).
meta-Fexofenadine (meta-MDL-1645; meta-MDL16455) is an impurity of Fexofenadine (Allegra; MDL-16455A; MDL16455A; Telfast), which is a histamine H1 receptor antagonist (antihistamine) approved for the treatment of allergy including hay fever, nasal congestion, and urticaria.
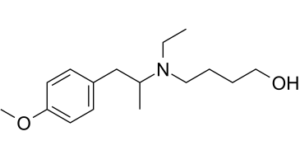
Mebeverine alcohol is a major metabolite of Mebeverine which is a musculotropic antispasmodic drug.
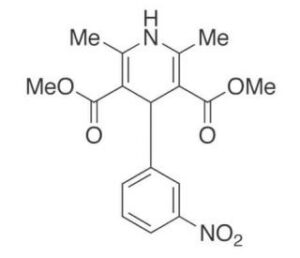
m-Nifedipine is an impurity of Nifedipine (Bay-1040; BAY-a-1040; Cordipin; Corinfar) which is an approved dihydropyridine calcium channel blocker (CCB) used as antihypertensive drug.
Oseltamivir carboxylate (GS-4071; Ro 64-0802) is a major and active metabolite of oseltamivir phosphate (Tamiflu).
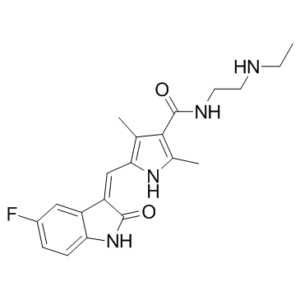
N-Desethyl Sunitinib (SU-11662) is a major metabolite of sunitinib which is a potent, ATP-competitive VEGFR, PDGFRβ and KIT inhibitor with Kis of 2, 9, 17, 8 and 4 nM for VEGFR -1, -2, -3, PDGFRβ and KIT, respectively.
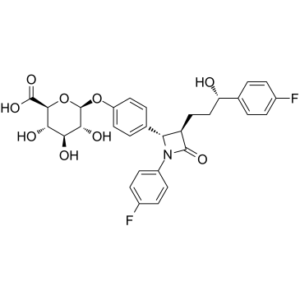
Ezetimibe phenoxy glucuronide (also called Ezetimibe glucuronide) is the active metabolite of Ezetimibe with antihyperlipoproteinemic activity.
2R,4R-Sacubitril is an impurity of Sacubitril (AHU377), which is an FDA approved medication acting as a NEP (neutral endopeptidase 24.11) inhibitor and used as a component of the heart failure medicine LCZ696.