This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 500mg | $430 | Check With Us |
| 1g | $600 | Check With Us |
| 5g | $1510 | Check With Us |
Cat #: V3158 CAS #: 1377049-84-7 Purity ≥ 98%
Description: Velpatasvir (formerly known as GS5816; GS-5816; VEL; trade name Vosevi) is a potent, selective, second-generation Hepatitis C virus NS5A protease inhibitor approved for clinical use with sofosbuvir in the treatment of hepatitis C infection of all six major genotypes. It inhibits hepatitis C viral replication through acting on the crucial 'membranous web' that facilitates RNA replication.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 883.00 |
|---|---|
| Molecular Formula | C49H54N8O8 |
| CAS No. | 1377049-84-7 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: >100 mg/mLr |
| Water: >100 mg/mLr | |
| Ethanol: >100 mg/mL | |
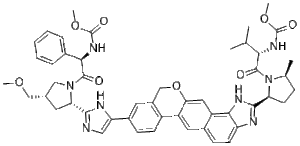
| SMILES Code | O=C(N([C@H]1C)[C@@H](CC1)C2=NC3=C(C(C=C(OCC4=CC(C5=CN=C([C@H](C[C@H](COC)C6)N6C ([C@@H](C7=CC=CC=C7)NC(OC)=O)=O)N5)=CC=C84)C8=C9)=C9C=C3)N2)[C@H](C(C)C)NC(OC)=O |
| Synonyms | GS5816; VEL; GS-5816; GS 5816; Vosevi |
| Protocol | In Vitro | In vitro activity: Velpatasvir (also known as GS-5816) is a novel pan-genotypic inhibitor of hepatitis C virus (HCV) nonstructural protein 5A (NS5A) with activity against genotype 1 (GT1) to GT6 HCV replicons. It is a selective inhibitor of HCV RNA replication with mean 50% effective concentrations (EC50s) against GT1 to GT6 of 6 to 130 pM. Kinase Assay: Velpatasvir (VEL, GS-5816) is a novel pan-genotypic hepatitis C virus (HCV) nonstructural protein 5A (NS5A) inhibitor with activity against genotype 1 (GT1) to GT6 HCV replicons. Cell Assay: Velpatasvir (VEL, GS-5816) is a novel pan-genotypic hepatitis C virus (HCV) nonstructural protein 5A (NS5A) inhibitor with activity against genotype 1 (GT1) to GT6 HCV replicons. In a phase 1b 3-day monotherapy study, patients treated with a 150-mg dose of GS-5816 had a mean maximal HCV RNA decline of ≥3.3 log10 IU/ml in GT1a, -1b, -2, -3, and -4. This report characterizes virologic resistance to VEL in these patients. NS5A resistance-associated substitutions (RASs) were detected by deep sequencing (1% cutoff) pretreatment in 22/70 patients, i.e., 10/35 (29%) patients with GT1a, 1/8 (13%) with GT1b, 4/8 (50.0%) with GT2, 5/17 (29.4%) with GT3, and 2/2 (100.0%) with GT4. In GT1a and GT3 patients, pretreatment RASs were associated with a slightly reduced HCV RNA response compared to that of patients without pretreatment RASs; among patients with GT1b, GT2, and GT4, no significant difference in response was observed in those with or without pretreatment RASs. Following treatment, the pattern of emergent RASs was more complex for GT1a than for the other genotypes. In GT1a, substitutions emerged at positions M28, Q30, L31, P32, H58, E92, and Y93, with the most prevalent substitutions at positions Y93, M28, and L31. RASs were observed at two positions in GT1b and GT2 (Y93 and L31), three positions in GT3 (Y93, L31, and E92), and four positions in GT4 (L28, M31, P32L, and Y93). RASs that were present pretreatment persisted through the 48-week follow-up period; however, RASs emerging during treatment were more likely to decline both in prevalence and in frequency within the viral population during follow-up. (This study has been registered at ClinicalTrials.gov under registration no. NCT01740791.). |
|---|
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.1325 mL | 5.6625 mL | 11.3250 mL | 22.6501 mL |
| 5mM | 0.2265 mL | 1.1325 mL | 2.2650 mL | 4.5300 mL |
| 10mM | 0.1133 mL | 0.5663 mL | 1.1325 mL | 2.2650 mL |
| 20mM | 0.0566 mL | 0.2831 mL | 0.5663 mL | 1.1325 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































