This product is for research use only, not for human use. We do not sell to patients.
| Size | Price |
|---|---|
| 250mg | Get quote |
| 500mg | Get quote |
| 1g | Get quote |
Cat #: V3249 CAS #: N/A Purity ≥ 99%
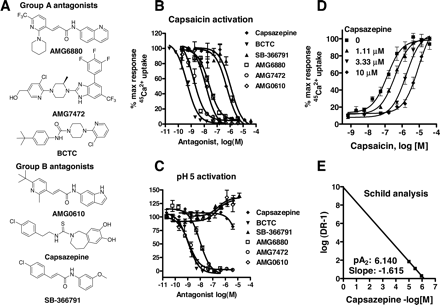
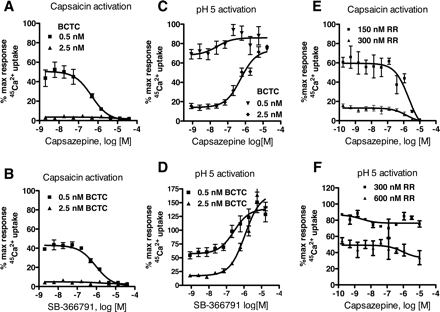
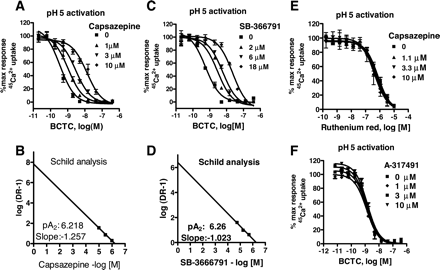
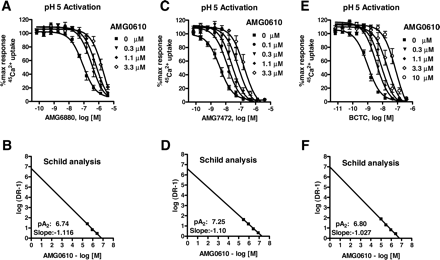
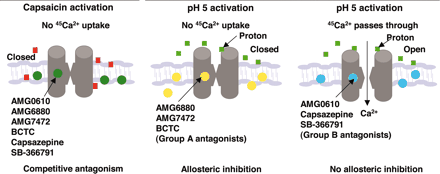
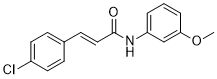
Description: SB-366791 analog is a novel, potent, competitive and selective cinnamide antagonist of the vanilloid receptor (VR1/TRPV1). TRPV1 is a non-selective cation channel, predominantly expressed by peripheral sensory neurones, which is known to play a key role in the detection of noxious painful stimuli, such as capsaicin, acid and heat. In a FLIPR-based Ca(2+)-assay, SB-366791 produced a concentration-dependent inhibition of the response to capsaicin with an apparent pK(b) of 7.74 +/- 0.08. Unlike capsazepine, SB-366791 was also an effective antagonist vs. the acid-mediated activation of rTRPV1. SB-366791 had a good selectivity profile exhibiting little or no effect in a panel of 47 binding assays and a variety of electrophysiological assays including hippocampal synaptic transmission and action potential firing of locus coeruleus or dorsal raphe neurones. SB-366791 may therefore prove to be a useful tool to further study the biology of TRPV1 and may also be developed as an antinociceptive drug.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| CAS No. | N/A |
|---|
| Protocol | In Vitro | In vitro activity: SB-366791 is a novel, potent, competitive and selective cinnamide antagonist of the vanilloid receptor (VR1/TRPV1) with IC50 of 5.7±1.2 nM. TRPV1 is a non-selective cation channel, predominantly expressed by peripheral sensory neurones, which is known to play a key role in the detection of noxious painful stimuli, such as capsaicin, acid and heat. In a FLIPR-based Ca(2+)-assay, SB-366791 produced a concentration-dependent inhibition of the response to capsaicin with an apparent pK(b) of 7.74 +/- 0.08. In electrophysiological experiments, SB-366791 was an effective antagonist of hTRPV1 when activated by different modalities, such as capsaicin, acid or noxious heat (50 degrees C). Unlike capsazepine, SB-366791 was also an effective antagonist vs. the acid-mediated activation of rTRPV1. SB-366791 had a good selectivity profile exhibiting little or no effect in a panel of 47 binding assays (containing a wide range of G-protein-coupled receptors and ion channels) and a variety of electrophysiological assays including hippocampal synaptic transmission and action potential firing of locus coeruleus or dorsal raphe neurones. Furthermore, unlike capsazepine, SB-366791 had no effect on either the hyperpolarisation-activated current (I(h)) or Voltage-gated Ca(2+)-channels (VGCC) in cultured rodent sensory neurones. In summary, SB-366791 is a new TRPV1 antagonist with high potency and an improved selectivity profile with respect to other commonly used TRPV1 antagonists. SB-366791 may therefore prove to be a useful tool to further study the biology of TRPV1. Kinase Assay: Capsaicin Antagonist Assay. compounds were preincubated with TRPV1 expressing CHO cells in Hanks' buffered saline solution supplemented with 0.1 mg/ml BSA and 1 mM HEPES at pH 7.4 at room temperature for 2 min before addition of 45Ca2+ and capsaicin (final concentration, 0.5 μM) in Ham's F-12 media and then left for an additional 2 min before compound washout. Cell Assay: 45Ca2+ Uptake Assay. Two days before the assay, cells were seeded in Cytostar 96-well plates (GE Healthcare, Little Chalfont, Buckinghamshire, UK) at a density of 20,000 cells/well. The activation of TRPV1 is followed as a function of cellular uptake of radioactive calcium (45Ca2+; MP Biomedicals, Irvine, CA). All the 45Ca2+ uptake assays had a final 45Ca2+ concentration at 10 μCi/ml. |
|---|---|---|
| In Vivo | Phα1β and SB366791 interact in a synergistic manner to cause antinociception. We found an interaction index (α) of 0.07 for Phα1β and SB366791 when these drugs were injected together intraplantarly, which indicates that in vivo interaction between these drugs is greater than additive interaction. Synergism also occurred when intraplantar SB366791 was administered simultaneously with intrathecal Phα1β (interaction index α=0.06) suggesting a 15 fold rise in potency on the analgesic effect of these drugs when they are added together. It was observed no significant alterations in body temperature of animals treated with this combination regimen. Swiss mice (20–25 g; 5–7 weeks old) were bred in our animal facility and housed at a controlled temperature (22 ± 2 °C) under a 12-h light/dark cycle with standard laboratory chow and tap water available ad libitum. The animals were habituated to the experimental room for at least 2 h prior to experiments. Each animal was used only once. The experiments reported in this study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications No. 8023, revised 1978) and ethical guidelines for the investigation of experimental pain in conscious animals. The Ethics Committee in experimentation with living animals from the Institute for Education and Research – Hospital Santa Casa, Belo Horizonte authorized all procedures (Protocol 002/2015). | |
| Animal model | Swiss mice (20–25 g; 5–7 weeks old) |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.