This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 5mg | $66 | 3-6 Days |
| 10mg | $98 | 3-6 Days |
| 25mg | $150 | 3-6 Days |
| 50mg | $245 | 3-6 Days |
| 100mg | $425 | 3-6 Days |
| 250mg | $750 | 3-6 Days |
| 500mg | $1250 | 3-6 Days |
Cat #: V3220 CAS #: 1378872-36-6 Purity ≥ 98%
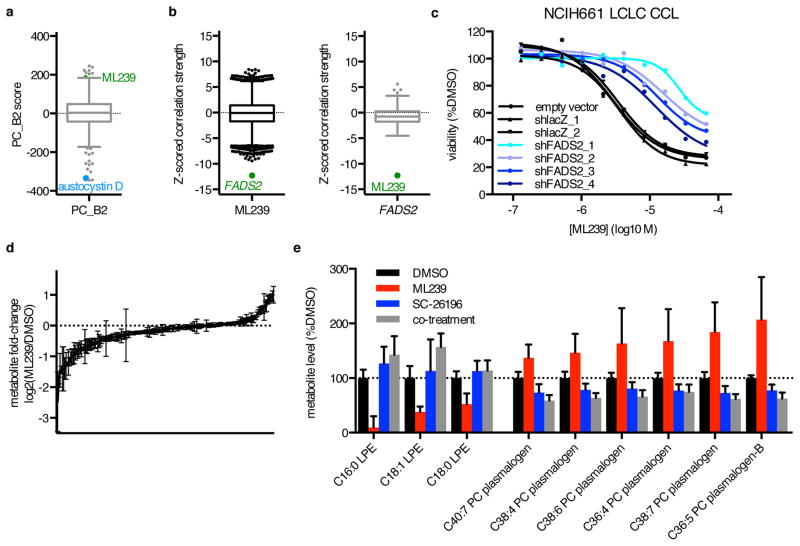
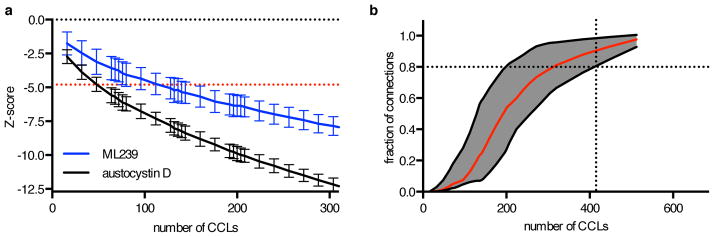
Description: ML239 was originally identified as a potent and selective inhibitor of breast cancer stem cells with an IC50 of 1.16 μM, but recent studies (2016 Nature 12(2):109-16.) suggested that it most likely acts through activation of fatty acid desaturase 2 (FADS2). ML239 was discovered from high-throughput screen (HTS) with the National Institute of Health–Molecular Libraries Small Molecule Repository (NIH–MLSMR) compound collection which identified a class of acyl hydrazones to be selectively lethal to breast cancer stem cell (CSC) enriched populations. Medicinal chemistry efforts were undertaken to optimize potency and selectivity of this class of compounds. The optimized compound was declared as a probe (ML239) with the NIH Molecular Libraries Program and displayed greater than 20-fold selective inhibition of the breast CSC-like cell line (HMLE_sh_Ecad) over the isogenic control line (HMLE_sh_GFP).
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 346.6 |
|---|---|
| Molecular Formula | C₁₃H₁₀Cl₃N₃O₂ |
| CAS No. | 1378872-36-6 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: ≥ 300 mg/mL |
| Water: N/A | |
| Ethanol: N/A | |
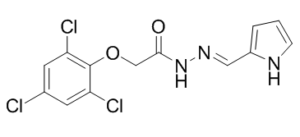
| SMILES Code | O=C(N/N=C/C1=CC=CN1)COC2=C(Cl)C=C(Cl)C=C2Cl |
| Synonyms | ML 239; ML-239; ML239 |
| Protocol | In Vitro | In vitro activity: ML239 was originally identified as a potent and selective inhibitor of breast cancer stem cells with an IC50 of 1.16 μM, but recent studies (2016 Nature 12(2):109-16.) suggested that it most likely acts through activation of fatty acid desaturase 2 (FADS2). ML239 was discovered from high-throughput screen (HTS) with the National Institute of Health–Molecular Libraries Small Molecule Repository (NIH–MLSMR) compound collection which identified a class of acyl hydrazones to be selectively lethal to breast cancer stem cell (CSC) enriched populations. Medicinal chemistry efforts were undertaken to optimize potency and selectivity of this class of compounds. The optimized compound was declared as a probe (ML239) with the NIH Molecular Libraries Program and displayed greater than 20-fold selective inhibition of the breast CSC-like cell line (HMLE_sh_Ecad) over the isogenic control line (HMLE_sh_GFP). Kinase Assay: ML239 is a potent and selective inhibitor of breast cancer stem cells, with an IC50 of 1.16 μM, with ∼24-fold selectivity against the control cell line. ML239 inhibits breast cancer stem-like cells, most likely through activation of fatty acid desaturase 2 (FADS2). ML239 is cytotoxic to NCIH661 cells, and FADS2 knockdown reduces ML239 cytotoxicity, and furthermore, FADS2 inhibitor SC-26196 also reduces ML239 cytotoxicity in cancer cell lines (CCLs). Cell Assay: Small molecules were selected individually to interrogate important targets and/or cellular processes in cancer with high reported selectivity, and collectively to target diverse nodes in cancer cell circuitry, from sources including FDA-approved drugs, clinical candidates, previous screening and sensitivity profiling experiments, scientific literature and patents, bioactives, and collaborator contributions. CCLs were plated at a density of 500 cells/well in white opaque tissue-culture-treated Aurora 1536-well MaKO plates (Brooks Automation) in the provider-recommended growth media using a highly automated platform. Compounds were added by acoustic transfer using a Labcyte Echo 555 (Labcyte Inc.) 24 hours after plating. The effects of small molecules were measured over a 16-point concentration range (two-fold dilution) in duplicate. DMSO was used at a constant concentration of 0.33%, including vehicle-only control wells. As a surrogate for viability, cellular ATP levels were assessed 72 hours after compound transfer by addition of CellTiterGlo (Promega) followed by luminescence measurement using a ViewLux Microplate Imager (PerkinElmer). Duplicates were averaged and luminescence values normalized to vehicle (DMSO) treatment and background (media-only) wells. |
|---|
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.8852 mL | 14.4259 mL | 28.8517 mL | 57.7034 mL |
| 5mM | 0.5770 mL | 2.8852 mL | 5.7703 mL | 11.5407 mL |
| 10mM | 0.2885 mL | 1.4426 mL | 2.8852 mL | 5.7703 mL |
| 20mM | 0.1443 mL | 0.7213 mL | 1.4426 mL | 2.8852 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.