This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 500mg | $750 | In Stock |
| 1g | $1000 | In Stock |
| 5g | $2530 | In Stock |
Cat #: V3509 CAS #: 1441674-54-9 Purity ≥ 98%
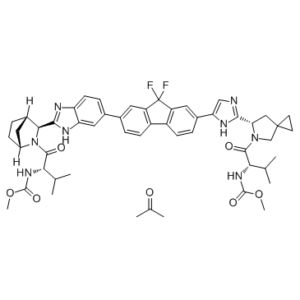
Description: Ledipasvir acetone (previously known as GS5885; GS-5885 acetone; Harvoni) is an HCV NS5A polymerase inhibitor with anti-HCV activity. It has been approved for for the treatment of hepatitis C virus (HCV) infection in combination with sofosbuvir (under the trade name of Harvoni for ledipasvir/sofosbuvir). Ledipasvir inhibits CV NS5A polymerase with EC50s of 34 pM and 4 pM against genotype 1a and 1b replicon, respectively.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 947.08 |
|---|---|
| Molecular Formula | C₅₂H₆₀F₂N₈O₇ |
| CAS No. | 1441674-54-9 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: > 30 mg/mLr |
| Water: N/Ar | |
| Ethanol: N/A | |
| SMILES Code | COC(N[C@H](C(N1CC2(C[C@H]1c3[nH]c(c4cc5c(c6c(C(F)5F)cc(c(cc7)cc8c7nc([C@@H]9[C@H]%10CC[C@@H](N9C([C@H](C(C)C)NC(OC)=O)=O)C%10)[nH]8)cc6)cc4)cn3)CC2)=O)C(C)C)=O.CC(C)=O |
| Synonyms | GS-5885 acetone, Ledipasvir acetone; GS5885 acetone; GS 5885; trade name: Harvoni; Methyl N-[(2S)-1-[(6S)-6-[5-[9,9-Difluoro-7-[2-[(1S,2S,4R)-3-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-3-azabicyclo[2.2.1]heptan-2-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate acetone Chemical Name: methyl ((S)-1-((S)-6-(5-(9,9-difluoro-7-(2-((1R,3S,4S)-2-((methoxycarbonyl)-L-valyl)-2-azabicyclo[2.2.1]heptan-3-yl)-1H-benzo[d]imidazol-6-yl)-9H-fluoren-2-yl)-1H-imidazol-2-yl)-5-azaspiro[2.4]heptan-5-yl)-3-methyl-1-oxobutan-2-yl)carbamate compound with propan-2-one (1:1) |
| Protocol | In Vitro | In vitro activity: Ledipasvir (also known as GS5885) is a HCV NS5A polymerase inhibitor that is used for the treatment of hepatitis C virus infection. The combination product of ledipasvir 90 mg/sofosbuvir 400 mg (trade name Harvoni) was approved by FDA in October 2014. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin. Ledipasvir has an extended plasma half-life of 37-45 h in healthy volunteers and produces a rapid >3 log viral load reduction in monotherapy at oral doses of 3 mg or greater with once-daily dosing in genotype 1a HCV-infected patients. It has been shown to be safe and efficacious, with SVR12 rates up to 100% when used in combination with direct-acting antivirals having complementary mechanisms. Kinase Assay: GT1a replicon EC50 = 31 pM Cell Assay: Ledipasvir is a specific inhibitor of HCV NS5A protein to inhibit HCV replication in the HCV subgenomic replicon system. NS5A replication complex inhibitors are novel antiviral factors for HCV treatment. Typically, these inhibitors have high efficiency and low viral resistance when compared to traditional HCV replication inhibitor targeted on NS3 helicase and NS5B RNA polymerasae. NS5A inhibitors are supposed to bind across the NS5A dimer interface, proximal to N-terminal domain 1. The binding is thought to distort dimer association directly or allosterically, which may disrupt NS5A function in HCV RNA replication. When a JFH1/3a-NS5A hybrid replicon was used to assess susceptibility to NS5A, another inhibitor DCV was shown to be more potent than ledipasvir. Additionally, NS5A-A30K and -Y93H variants exhibited reduced sensitivity to ledpasvir (EC50 value of 1770 nM and 4300 nM respectively). |
|---|---|---|
| In Vivo | In clinical trials, it was observed ledpasvir was well tolerated and exhibited median maximal reduction of HCV RNA ranging from 2.3 log10 IU/ml to 3.3 log10 IU/ml. Emax modeling also showed administration of 30 mg ledpasvir after 3 days resulted in >95% maximal response of HCV RNA reduction to genotype 1a.Finally, it was also observed that HCV RNA was more sustained in genotype 1b compared to 1a. | |
| Animal model | PK studies in Rats, Dogs and Monkeys; Ledipasvir is remarkable not only on the basis of its high replicon potency but also on the basis of its low clearance, good bioavailability, and long half-lives in rat, dog, and monkey and low predicted clearance in human. The pharmacokinetics of Ledipasvir is measured in rats and dogs. Ledipasvir shows good half-lives (rat 1.83 ± 0.22 hr, dog 2.63 ± 0.18 hr) in plasma, low systemic clearance (CL), and moderate volumes of distribution (Vss) that are greater than total body water volume |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.0559 mL | 5.2794 mL | 10.5588 mL | 21.1175 mL |
| 5mM | 0.2112 mL | 1.0559 mL | 2.1118 mL | 4.2235 mL |
| 10mM | 0.1056 mL | 0.5279 mL | 1.0559 mL | 2.1118 mL |
| 20mM | 0.0528 mL | 0.2640 mL | 0.5279 mL | 1.0559 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































