This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 250mg | $700 | Check With Us |
| 500mg | $1250 | Check With Us |
| 1g | $1875 | Check With Us |
Cat #: V3160 CAS #: 1350462-55-3 Purity ≥ 98%
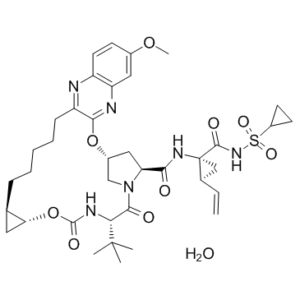
Description: Grazoprevir hydrate (previously known as MK 5172; MK-5172; MK5172; Zepatier), the mono-hydrated form of grazoprevir, is a second generation inhibitor of HCV (Hepatitis C Virus) NS3/4A Protease and a marketed broad spectrum anti-HCV drug with activity across genotypes and resistant variants. It inhibits HCV genotype 1a, 1B, and 4 with IC50 values of 7pM, 4pM, and 62pM, respectively. In 2016, Grazoprevir was approved by FDA for the treatment of hepatitis C along with the NS5A replication complex inhibitor elbasvir, either with or without ribavirin.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 784.93 |
|---|---|
| Molecular Formula | C38H52N6O10S |
| CAS No. | 1350462-55-3 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: >100 mg/mLr |
| Water: < 1 mg/mLr | |
| Ethanol: >100 mg/mL | |
| SMILES Code | COC1=CC2=C(N=C(CCCCC[C@@H]3C[C@H]3OC4=O)C(O[C@H]5CN(C([C@H](C(C)(C)C)N4)=O)[C@H](C(N[C@@]([C@@H]6C=C)(C6)C(NS(C7CC7)(=O)=O)=O)=O)C5)=N2)C=C1.O |
| Synonyms | MK5172 hydrate; MK 5172; MK-5172 hydrate, Trade name: Zepatier. |
| Protocol | In Vitro | In vitro activity: MK-5172 (Grazoprevir) is effective in biochemical assays against major genotypes and variants engineered with common resistant mutations, with Ki of 0.01±<0.01 nM (gt1b), 0.01±0.01 nM (gt1a), 0.08±0.02 nM (gt2a), 0.15±0.06 nM (gt2b), 0.90±0.2 nM (gt3a), 0.07±0.01 nM (gt1bR155K), 0.14±0.03 nM (gt1bD168V), 0.30±0.04 nM (gt1bD168Y), 5.3±0.9 nM (gt1bA156T), and 12±2 nM (gt1bA156V), respectively. In the replicon assay, MK-5172 demonstrates subnanomolar to low-nanomolar EC50s against genotypes 1a, 1b, and 2a, with EC50s of 0.5±0.1 nM, 2±1 nM, and 2±1 nM for gt1bcon1, gt1a, and gt2a, respectively. MK-5172 is potent against a panel of HCV replication mutants NS5A (Y93H) (EC50=0.7±0.3 nM), NS5B nucleosides (S282T) (EC50=0.3±0.1 nM), and NS5B (C316Y) (EC50=0.4±0.2). MK-5172 maintains the excellent potency against the gt 3a enzyme as well as a broad panel of mutant enzymes, has excellent potency in the replicon system [gt1b IC50(50% NHS)=7.4 nM; gt1a IC50(40% NHS)=7 nM], and shows excellent rat liver exposure. Kinase Assay: recombinant HCV NS3/4A enzymes are expressed and purified from E. Coli. Enzyme sequences are derived from genotype 1a (gt1a) H77, gt1b con1, gt2a JFH1, gt2b HCJ8, and gt3a NZL1. Inhibition of HCV NS3/4A protease activity in reaction mixtures containing MK-5172 (Grazoprevir), Vaniprevir, or the reference compounds Danoprevir and TMC435 is determined in a time-resolved fluorescence assay. Cell-based HCV replicon assays are conducted in genotype 1b (con1) stable cell line HB1 or a gt2a cell line (JFH) in the presence of either 10% fetal bovine serum (FBS) or 40% normal human serum (NHS). Determinations of 50% effective concentrations (EC50s) against the panel of genotype or mutant replicon cell lines are conducted using a TaqMan-based assay. The 50% cytotoxic concentration (CC50) is determined in the HCV replicon cell line with the use of an MTS assay. Potency determinations against clinical genotype 1 NS3/4A sequences are made using a transient cell-based phenotype assay. The NS3/4A patient isolates are cloned from human plasma infected with HCV. Broad counterscreening, in which MK-5172 is evaluated for its inhibitory potency at a concentration of 10 μM, is conducted at MDS Pharma Services. Cell Assay: HB1 cells (30,000 per well) are seeded of a 6-well tissue culture plate per drug concentration. The next day (day 0), the medium is replenished with fresh medium and MK-5172 at the appropriate drug concentration. Cells from a single well per drug concentration are harvested on days 0, 1, and 2, washed, and stored frozen until evaluation. The fourth well is similarly harvested on day 3.5 except that 30,000 cells are reseeded with fresh medium and MK-5172 at the appropriate drug concentration. For additional time points, cells are passaged and harvested every one-half week for 2 weeks. For the third week, cells are similarly treated except that cells received replenishing medium which contained 0.5 mg/ml G418 without protease inhibitor. |
|---|---|---|
| In Vivo | MK-5172 (Grazoprevir) demonstrates high in vivo efficacy against chronic-HCV-infected chimpanzees. When dosed to dogs, MK-5172 shows low clearance of 5 mL/min/kg and a 3 h half-life after iv dosing and has good plasma exposure (AUC=0.4 μM h) after a 1 mg/kg oral dose. Dog liver biopsy studies showed that the liver concentration of MK-5172 after the 1 mg/kg oral dose is 1.4 μM at the 24 h time point. Similar to its behavior in rats, MK-5172 demonstrates effective partitioning into liver tissue and maintains high liver concentration, relative to potency, 24 h after oral dosing in dogs. | |
| Animal model | Rat and dog | |
| Formulation | Formulated in polyethylene glycol 200 (PEG200) | |
| Dosages | Administered as a bolus at either 2 mg/kg of body weight (Rats) or 0.5 mg/kg (dog). For oral studies, the crystalline potassium salt of the compound is dosed as a solution in PEG400 at 5 mg/kg (Rats) or 1 mg/kg (dog). |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.2740 mL | 6.3700 mL | 12.7400 mL | 25.4800 mL |
| 5mM | 0.2548 mL | 1.2740 mL | 2.5480 mL | 5.0960 mL |
| 10mM | 0.1274 mL | 0.6370 mL | 1.2740 mL | 2.5480 mL |
| 20mM | 0.0637 mL | 0.3185 mL | 0.6370 mL | 1.2740 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































