This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 5mg | $100 | 3-6 Days |
| 10mg | $150 | 3-6 Days |
| 25mg | $250 | 3-6 Days |
| 50mg | $415 | 3-6 Days |
| 100mg | $619 | 3-6 Days |
| 250mg | $1050 | 3-6 Days |
| 500mg | $1550 | 3-6 Days |
Cat #: V2977 CAS #: 863513-91-1 Purity ≥ 98%
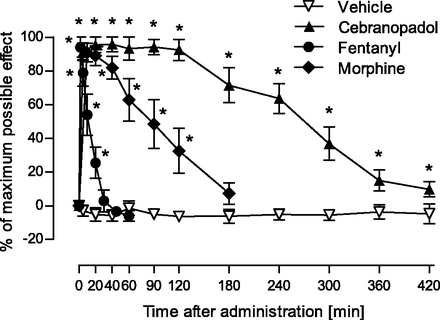
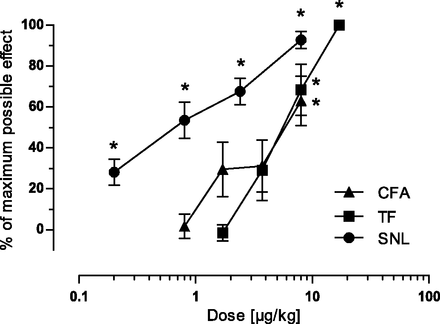
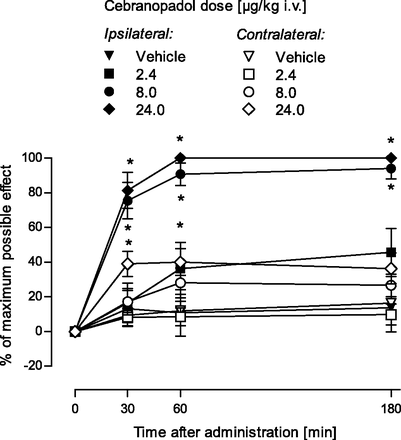
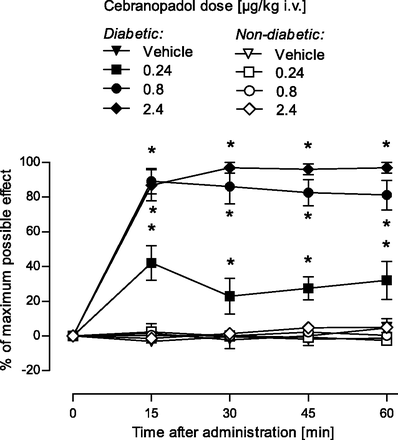
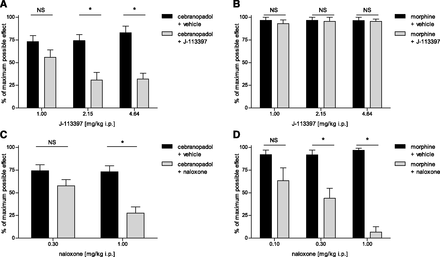
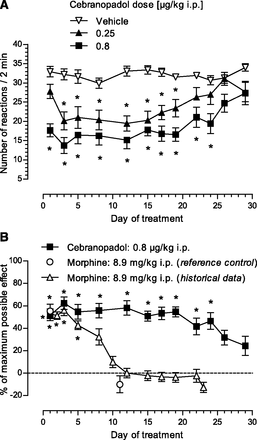
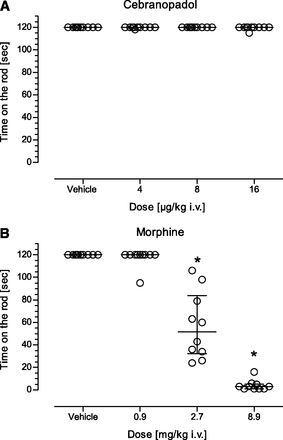
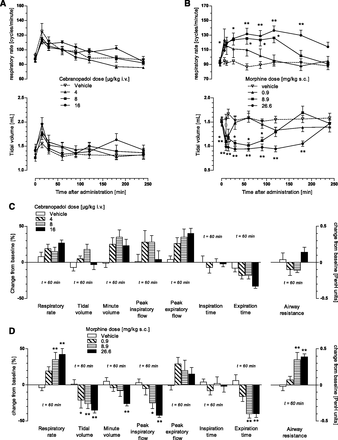
Description: Cebranopadol (also known as GRT-6005) is a novel, first in class compound with potent agonist activity on ORL-1 (opioid receptor like -1) and the well established mu opioid receptor. Cebranopadol is an analgesic nociceptin/orphanin FQ peptide (NOP) that exhibits high potency and efficacy in several rat models of acute and chronic pain (tail-flick, rheumatoid arthritis, bone cancer, spinal nerve ligation, diabetic neuropathy) with ED50 values of 0.5-5.6 µg/kg after intravenous and 25.1 µg/kg after oral administration. It is being evaluated in clinical Phase 2 and Phase 3 trials for the treatment of chronic and acute pain. Recent evidence indicates that the combination of opioid and NOP receptor agonism may be a new treatment strategy for cocaine addiction.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 378.49 |
|---|---|
| Molecular Formula | C24H27FN2O |
| CAS No. | 863513-91-1 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: >10mM |
| Water: <1 mg/mL | |
| Ethanol: | |
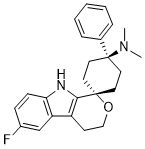
| SMILES Code | CN(C)[C@]1(C2=CC=CC=C2)CC[C@]3(C(N4)=C(CCO3)C5=C4C=CC(F)=C5)CC1 |
| Synonyms | GRT6005; GRT 6005; GRT-6005; Cebranopadol |
| Protocol | In Vitro | In vitro activity: Cebranopadol (also known as GRT-6005) is a novel, first in class compound with potent agonist activity on ORL-1 (opioid receptor like -1) and the well established mu opioid receptor. Cebranopadol is an analgesic nociceptin/orphanin FQ peptide (NOP) that exhibits high potency and efficacy in several rat models of acute and chronic pain (tail-flick, rheumatoid arthritis, bone cancer, spinal nerve ligation, diabetic neuropathy) with ED50 values of 0.5-5.6 µg/kg after intravenous and 25.1 µg/kg after oral administration. It is being evaluated in clinical Phase 2 and Phase 3 trials for the treatment of chronic and acute pain. Recent evidence indicates that the combination of opioid and NOP receptor agonism may be a new treatment strategy for cocaine addiction. Kinase Assay: Human MOP, DOP, KOP, and NOP receptor binding assays were run in microtiter plates (Costar 3632; Corning Life Sciences, Tewksbury, MA) with wheat germ agglutinin-coated scintillation proximity assay beads (GE Healthcare, Chalfont St. Giles, UK). Cell membrane preparations of Chinese hamster ovary K1 cells transfected with the human MOP receptor (Art.-No. RBHOMM, lot-No. 307-065-A) or the human DOP receptor (Art.-No. RBHODM, lot-No. 423-553-B), and human embryonic kidney cell line 293 cells transfected with the human NOP receptor (Art.-No. RBHORLM, lot-No. 1956) or the human KOP receptor (Art.-No. 6110558, lot-No. 295-769-A) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). [N-allyl-2,3-3H]naloxone and [tyrosyl-3,5-3H]deltorphin II (both purchased from PerkinElmer Life and Analytical Sciences), [3H]Ci-977, and [leucyl-3H]nociceptin (both purchased from GE Healthcare) were used as ligands for the MOP, DOP, KOP, and NOP receptor binding studies, respectively. Cell Assay: To test the agonistic activity of cebranopadol on human recombinant MOP, DOP, or NOP receptor-expressing cell membranes from Chinese hamster ovary K1 cells, or KOP receptor-expressing cell membranes from human embryonic kidney cell line 293 cells, 10 µg of membrane proteins per assay was incubated with 0.4 nM [35S]GTPγS (GE Healthcare) and different concentrations of agonists in buffer containing 20 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 1.28 mM NaN3, and 10 µM guanosine diphosphate for 45 minutes at 25°C. The bound radioactivity was determined as previously described. |
|---|---|---|
| In Vivo | Behavioral studies in pain models and pharmacokinetic evaluations were conducted in Sprague-Dawley rats (weight range 134−423 g; tail-flick model: Iffa Credo, Brussels, Belgium; bone cancer model: Harlan Laboratories, Indianapolis, IN; all other pain models and pharmacokinetics: Janvier Laboratories, Le Genest Saint Isle, France); male rats were used for most of the experiments, except for the tail-flick and bone cancer models, for which female Sprague-Dawley rats were used. Studies in side effect models were conducted in male Wistar rats (weight range 150−375 g; Dépré, Saint Doulchard, France). Rats were housed under standard conditions (room temperature 20−24°C, 12 hour light/dark cycle, relative air humidity 35−70%, 10−15 air changes per hour, air movement<0.2 m/s) with food and water available ad libitum in the home cage. Animals were used only once in all in vivo models, except for models of mononeuropathy, for which they were tested repeatedly with a washout period of at least 1 week between tests. Apart from the exceptions mentioned below, animal testing was performed in accordance with the recommendations and policies of the International Association for the Study of Pain and the German Animal Welfare Law. All study protocols were approved by the local government committee for animal research, which is advised by an independent ethics committee. Animals were assigned randomly to treatment groups. Different doses and vehicles were tested in a randomized fashion. Although the operators performing the behavioral tests were not formally 'blinded' with respect to the treatment, they were not aware of the study hypothesis or the nature of differences between drugs. | |
| Animal model | Sprague-Dawley rats |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.6421 mL | 13.2104 mL | 26.4208 mL | 52.8416 mL |
| 5mM | 0.5284 mL | 2.6421 mL | 5.2842 mL | 10.5683 mL |
| 10mM | 0.2642 mL | 1.3210 mL | 2.6421 mL | 5.2842 mL |
| 20mM | 0.1321 mL | 0.6605 mL | 1.3210 mL | 2.6421 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.