This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 100mg | $900 | Check With Us |
| 250mg | $1550 | Check With Us |
| 500mg | $2325 | Check With Us |
Cat #: V3279 CAS #: 841301-32-4 Purity ≥ 98%
Description: Amenamevir (formerly ASP2151; ASP-2151) is a HSV (herpes simplex virus) helicase-primase inhibitor with potent antiviral activity against HSVs with EC50 of 14 ng/mL. It an antiviral drug used for the treatment of shingles, acting as an inhibitor of the zoster virus's helicase–primase complex. Amenamevir was approved in Japan for the treatment of shingles in 2017. Amenamevir a novel class of antiviral agent other than nucleoside compounds, such as aciclovir, valaciclovir and famciclovir. Amenamevir 400 and 200 mg were well tolerated as well as valaciclovir. The proportions of patients who experienced drug-related adverse events were 10.0% (25/249), 10.7% (27/252) and 12.0% (30/249) with amenamevir 400 and 200 mg and valaciclovir, respectively. In conclusion, amenamevir 400 mg appears to be effective and well tolerated for treatment of herpes zoster in immunocompetent Japanese patients.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 482.55 |
|---|---|
| Molecular Formula | C24H26N4O5S |
| CAS No. | 841301-32-4 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: >150 mg/mLr |
| Water: NAr | |
| Ethanol: NA | |
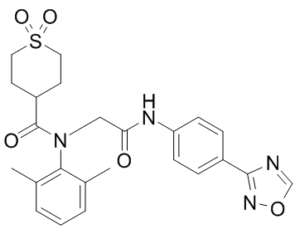
| SMILES Code | O=C(C(CC1)CCS1(=O)=O)N(C2=C(C)C=CC=C2C)CC(NC3=CC=C(C4=NOC=N4)C=C3)=O |
| Synonyms | ASP-2151; ASP2151; ASP 2151 |
| Protocol | In Vitro | In vitro activity: Amenamevir a novel class of antiviral agent other than nucleoside compounds, such as aciclovir, valaciclovir and famciclovir. ASP2151 inhibited the in vitro replication of HSV-1 with a mean 50% effective concentration (EC(50)) of 14 ng/ml. Kinase Assay: Amenamevir (formerly known as ASP2151) is a HSV (herpes simplex virus) helicase-primase inhibitor with potent antiviral activity against HSVs with EC50 of 14 ng/mL. Cell Assay: The antiviral activities of Amenamevir (ASP2151) and ACV against HSVs are tested using a plaque reduction assay. Briefly, HEF cells are seeded into multi well plates and incubated until they form a monolayer. After the medium is removed, the cells are infected with HSV-1 or HSV-2, and the plates are further incubated for 1 h at 37°C. The cells are washed twice with maintenance medium and then treated with the test compound (including Amenamevir) until clear plaques appear. The cells are then fixed with 10% formalin in phosphate-buffered saline, stained with a 0.02% crystal violet solution, and the number of plaques is determined under a light microscope. The EC50, which represents the concentration of test compound needed to reduce the plaque number by 50%, is calculated using nonlinear regression analysis with a sigmoid-maximum effect (Emax) model. |
|---|---|---|
| In Vivo | In the cutaneously HSV-1-infected mouse model, ASP2151 dose dependently suppressed intradermal HSV-1 growth, with the effect reaching a plateau at a dose of 30 mg/kg of body weight/day. The dose fractionation study showed that intradermal HSV-1 titers were below the detection limit in mice treated with ASP2151 at 100 mg/kg/day divided into two daily doses and at 30 or 100 mg/kg/day divided into three daily doses. The intradermal HSV-1 titer correlated with the maximum concentration of drug in serum (C(max)), the area under the concentration-time curve over 24 h (AUC(24h)), and the time during which the concentration of ASP2151 in plasma was above 100 ng/ml (T(>100)). The continuous infusion of ASP2151 effectively decreased intradermal HSV-1 titers below the limit of detection in mice in which the ASP2151 concentration in plasma reached 79 to 145 ng/ml. Our findings suggest that the antiviral efficacy of ASP2151 is most closely associated with the PK parameter T(>100) in HSV-1-infected mice. Based on these results, we propose that a plasma ASP2151 concentration exceeding 100 ng/ml for 21 to 24 h per day provides the maximum efficacy in HSV-1-infected mice. | |
| Animal model | Female hairless mice (HOS:HR-1, 7 to 8 weeks old) with HSV-1-infection | |
| Formulation | Formulated in polyethylene glycol 400 [PEG 400] | |
| Dosages | 1, 3, 10, 30, or 100 mg/kg/day | |
| Administration | Orally administered to HSV-1-infected mice (n=5) for 5 days |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.0723 mL | 10.3616 mL | 20.7232 mL | 41.4465 mL |
| 5mM | 0.4145 mL | 2.0723 mL | 4.1446 mL | 8.2893 mL |
| 10mM | 0.2072 mL | 1.0362 mL | 2.0723 mL | 4.1446 mL |
| 20mM | 0.1036 mL | 0.5181 mL | 1.0362 mL | 2.0723 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































