This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 100mg | $50 | 3-6 Days |
| 250mg | $70 | 3-6 Days |
| 500mg | $100 | 3-6 Days |
| 1g | $150 | 3-6 Days |
| 25g | $900 | 3-6 Days |
Cat #: V1652 CAS #: 17440-83-4 Purity ≥ 98%
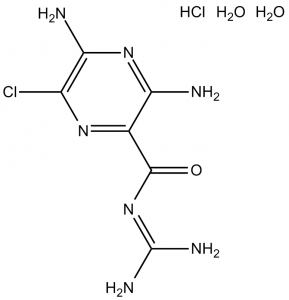
Description: Amiloride HCl dihydrate (MK870; MK-870; MK 870; Midamor; Midoride; Modamide), the hydrochloride salt and dihydrated form of amiloride, is a potent and selective epithelial sodium channel (ENaC) blocker that has been used since 1967 in the management of hypertension and congestive heart failure.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 302.12 |
|---|---|
| Molecular Formula | C6H8ClN7O.HCl.2H2O |
| CAS No. | 17440-83-4 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 60 mg/mL (198.6 mM) |
| Water: <1 mg/mL | |
| Ethanol: <1 mg/mL | |
| Synonyms | MK870; Amiloride Hydrochloride; MK-870; MK 870; Midamor; Midoride; Modamide. |
| Protocol | In Vitro | In vitro activity: Amiloride also induces the dephosphorylation of P13K (phosphatidylinositol 3-kinase) and PDK-1 (phosphoinositide-dependent kinase-1) kinases along with PTEN (phosphatase and tensin homolog deleted on chromosome 10) and PP1 alpha phosphatases. Amiloride inhibits phosphorylation of kinases and phosphatases by competing with ATP. Amiloride, which causes little or no cytotoxicity by itself, enhances TRAIL-induced apoptosis. Amiloride precludes the alkalinization and in parallel inhibit cellular proliferation. Amiloride directly inhibits autophosphorylation of the EGF receptor. Amiloride significantly enhances recovery to a maximum of 39%, 88%, and 78% for force, +dF/dt, and -dF/dt, respectively. Amiloride, a frequently used inhibitor of Na+/H+ exchange, rapidly inhibits phorbol ester-stimulated protein phosphorylation in vivo and protein kinase C-mediated phosphorylation in vitro, both with potency similar to that with which Amiloride inhibits Na+/H+ exchange. Amiloride blocks phorbol ester-induced adhesion of HL-60 cells (adhesion being a property indicative of the differentiated state), but dimethylamiloride (as well as ethylisopropylamiloride, another very potent amiloride analog) does not. Amiloride inhibits the ouabain-sensitive rate of oxygen consumption (QO2) of a suspension of rabbit intact proximal tubules in the presence of different concentrations of extracellular sodium. Cell Assay: Amiloride blocks δβγ channels with an IC50 of 2.6 μM (58, 71, 75, 134, 148). The Ki of amiloride for δβγ ENaC is 26-fold that of αβγ channels (0.1 μM for αβγ ENaC). Amiloride blockade of δβγ ENaC is much more voltage dependent compared with the αβγ channel. The Ki of amiloride for δαβγ channels is 920 and 13.7 μM at -120 and +80 mV, respectively, which significantly differs from that of both αβγ and δβγ channels. Amiloride is a relatively selective inhibitor of the epithelial sodium channel (ENaC) with an IC50 (the concentration required to reach 50% inhibition of an ion channel) in the concentration range of 0.1 to 0.5 μM. Amiloride is a relatively poor inhibitor of the the Na+/H+ exchanger (NHE) with an IC50 as low as 3 μM in the presence of a low external [Na+] but as high as 1 mM in the presence of a high [Na+]. Amiloride is an even weaker inhibitor of the Na+/Ca2+ exchanger (NCX), with an IC50 of 1 mM. Amiloride (1 μM) and submicromolar doses of Benzamil (30 nM), doses known to inhibit the ENaC, inhibit the myogenic vasoconstriction response to increasing perfusion pressure by blocking the activity of ENaC proteins. Amiloride completely inhibits Na+ influx in doses known to be relatively specific for ENaC (1.5 μM) in vascular smooth muscle cells (VSMC). |
|---|---|---|
| In Vivo | Amiloride (1 mg/kg/day) subcutaneously is found to reverse the initial increases in collagen deposition and prevent any further increases in the DOCA-salt hypertensive rat. Amiloride delays the onset of proteinuria and improved brain and kidney histologic scores in the saline-drinking, stroke-prone spontaneously hypertensive rats (SHRSP) compared with controls. Amiloride antagonizes or prevents actions of aldosterone in these cells and in cardiovascular and renal tissues in animals with salt-dependent forms of hypertension. | |
| Animal model | Rats |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 3.3099 mL | 16.5497 mL | 33.0994 mL | 66.1989 mL |
| 5mM | 0.6620 mL | 3.3099 mL | 6.6199 mL | 13.2398 mL |
| 10mM | 0.3310 mL | 1.6550 mL | 3.3099 mL | 6.6199 mL |
| 20mM | 0.1655 mL | 0.8275 mL | 1.6550 mL | 3.3099 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































