This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 250mg | $1000 | Check With Us |
| 500mg | $1750 | Check With Us |
| 1g | $2625 | Check With Us |
Cat #: V3171 CAS #: 1365970-03-1 Purity ≥ 98%
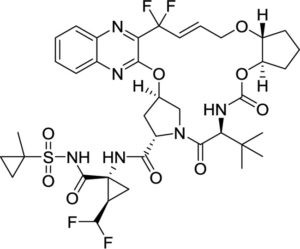
Description: Glecaprevir (previously called ABT493; A1282576; ABT-493; A-1282576; Mavyret) is a novel and potent inhibitor of the hepatitis C virus nonstructural protein 3/4A protease (HCV NS3/4A) approved as anti-HCV drug in co-formulation with an HCV NS5A inhibitor pibrentasvir. It inhibits HCV NS3/4A protease with IC50 ranging from 3.5 to 11.3 nM. In August 2017, the U.S. Food and Drug Administration approved the combination drug glecaprevir/pibrentasvir (trade name Mavyret) for the treatment of all major genotypes (1–6) of chronic hepatitis C.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 838.87 |
|---|---|
| Molecular Formula | C38H46F4N6O9S |
| CAS No. | 1365970-03-1 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: ≥ 80 mg/mLr |
| Water: N/Ar | |
| Ethanol: N/A | |
| SMILES Code | O=C([C@H]1N(C([C@@H](NC(O[C@@]2([H])[C@@]3([H])CCC2)=O)C(C)(C)C)=O)C[C@@](OC4=NC5=CC=CC=C5N=C4C(F)(/C=C/CO3)F)([H])C1)N[C@]6([C@H](C(F)F)C6)C(NS(=O)(C7(CC7)C)=O)=O |
| Synonyms | ABT493; Mavyret; A1282576; ABT-493; A-1282576; ABT 493; A 1282576 |
| Protocol | In Vitro | In vitro activity: Glecaprevir (formerly ABT-493) is a novel hepatitis C virus (HCV) NS3/4A protease inhibitor (PI) with pangenotypic activity. It inhibited the enzymatic activity of purified NS3/4A proteases from HCV genotypes 1 to 6 in vitro (half-maximal [50%] inhibitory concentration = 3.5 to 11.3 nM) and the replication of stable HCV subgenomic replicons containing proteases from genotypes 1 to 6 (50% effective concentration [EC50] = 0.21 to 4.6 nM). Glecaprevir had a median EC50 of 0.30 nM (range, 0.05 to 3.8 nM) for HCV replicons containing proteases from 40 samples from patients infected with HCV genotypes 1 to 5. Importantly, glecaprevir was active against the protease from genotype 3, the most-difficult-to-treat HCV genotype, in both enzymatic and replicon assays demonstrating comparable activity against the other HCV genotypes. In drug-resistant colony selection studies, glecaprevir generally selected substitutions at NS3 amino acid position A156 in replicons containing proteases from genotypes 1a, 1b, 2a, 2b, 3a, and 4a and substitutions at position D/Q168 in replicons containing proteases from genotypes 3a, 5a, and 6a. Although the substitutions A156T and A156V in NS3 of genotype 1 reduced susceptibility to glecaprevir, replicons with these substitutions demonstrated a low replication efficiency in vitro Glecaprevir is active against HCV with most of the common NS3 amino acid substitutions that are associated with reduced susceptibility to other currently approved HCV PIs, including those at positions 155 and 168. Combination of glecaprevir with HCV inhibitors with other mechanisms of action resulted in additive or synergistic antiviral activity. In summary, glecaprevir is a next-generation HCV PI with potent pangenotypic activity and a high barrier to the development of resistance. Kinase Assay: Glecaprevir inhibited the enzymatic activity of HCV genotype 1 to 6 NS3/4A proteases, with the half-maximal (50%) inhibitory concentration (IC50) values ranging from 3.5 to 11.3 nM in a biochemical assay. When glecaprevir was tested against six human serine proteases (chymase, chymotrypsin type II, chymotrypsin type VII, elastase, kallikrein, and urokinase) and one human cysteine protease (cathepsin B), no inhibition was observed at concentrations up to 200,000 nM. These results indicate that glecaprevir demonstrates a high level of selectivity for the HCV NS3/4A protease over the human proteases tested. Cell Assay: HCV subgenomic replicon cells containing the protease from genotype 1a, 1b, 2a, 2b, 3a, 4a, 5a, or 6a were passaged in the presence of glecaprevir at concentrations 10- or 100-fold its EC50s for the respective replicon cell lines to select for colonies with substitutions that conferred resistance to glecaprevir. Glecaprevir generally selected a low number of colonies containing resistance-conferring substitutions in replicon cells across the different HCV genotypes. Higher colony counts were observed with selections using replicons with the genotype 2a JFH-1 backbone (i.e., the genotype 2a and 2b replicons in this study), regardless of the selection concentrations. This was likely due to the exceptionally high replication rates for replicons with this backbone. |
|---|
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.1921 mL | 5.9604 mL | 11.9208 mL | 23.8416 mL |
| 5mM | 0.2384 mL | 1.1921 mL | 2.3842 mL | 4.7683 mL |
| 10mM | 0.1192 mL | 0.5960 mL | 1.1921 mL | 2.3842 mL |
| 20mM | 0.0596 mL | 0.2980 mL | 0.5960 mL | 1.1921 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































