This product is for research use only, not for human use. We do not sell to patients.
| Size | Price |
|---|---|
| 250mg | Get quote |
| 500mg | Get quote |
| 1g | Get quote |
Cat #: V3708 CAS #: 856867-39-5 Purity ≥ 98%
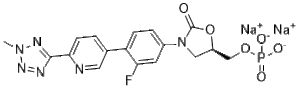
Description: Tedizolid phosphate disodium (DA-7157; TR-700; DA7157; TR700; torezolid; Sivextro) is the disodium salt of Tedizolid phosphate which is the phosphate ester prodrug of tedizolid. Tedizolid was approved in 2014 by FDA to treat acute bacterial skin and skin structure infections.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 494.29 |
|---|---|
| Molecular Formula | C17H14FN6Na2O6P |
| CAS No. | 856867-39-5 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: ≥ 37 mg/mL (80 mM) |
| Water: ≥ 1 mg/mL | |
| Ethanol: ≥ 1 mg/mL | |
| Synonyms | Tedizolid phosphate disodium salt; Torezolid phosphate sodium salt; DA 7218 phosphate sodium salt; TR 701 phosphate sodium salt; TR-701 phosphate sodium salt |
| Protocol | In Vitro | In vitro activity: Tedizolid (formerly known as torezolid, TR-700, or DA-7157, trade name Sivextro), is an oxazolidinone-class antibiotic against Gram-positive bacteria. The mechanism of action is to inhibit protein synthesis by binding to the 50S ribosomal subunit of the G+ bacteria. Tedizolid phosphate is a phosphate ester prodrug of the active compound tedizolid. It was developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics (formerly Dong-A Pharmaceuticals in Korea), and is approved in 2014 by the US-FDA for the treatment of acute bacterial skin and skin structure infections (also known as complicated skin and skin-structure infections (cSSSIs)). Kinase Assay: Tedizolid (0.25 μg/mL) inhibits all 28 clinical isolates of PRSP, and is 4-fold more potent than linezolid against PRSP Cell Assay: Tedizolid (0.25 μg/mL) inhibits all 28 clinical isolates of PRSP, and is 4-fold more potent than linezolid against PRSP |
|---|---|---|
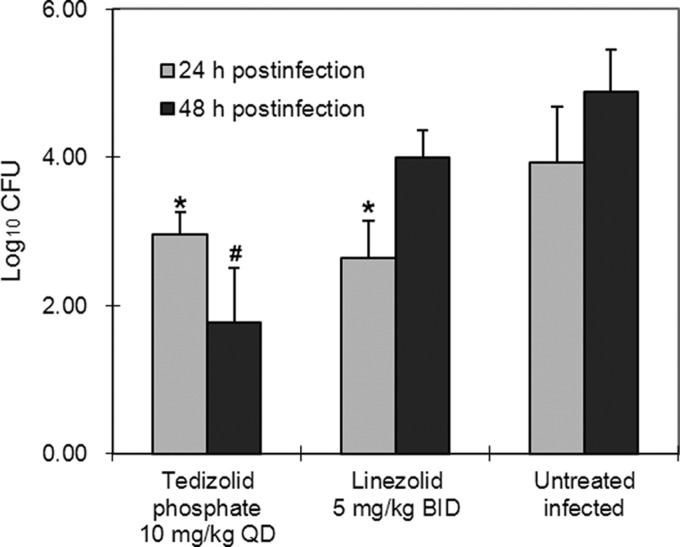
| In Vivo | Male ICR mice (weight, 18 to 20 g) are inoculated intraperitoneally with 1 of 4 PRSP isolates (DR9, DR10, DR11, or DR14) suspended in 10% mucin, to induce a systemic S. pneumoniae infection. The suspension contained sufficient bacteria to kill 100% of untreated control mice. At 1 h postinfection, mice receives a single dose of either tedizolid phosphate or linezolid, and survival is assessed daily for 7 days postinfection. Treatments are delivered both orally and intravenously at each of four doses (40 mg/kg of body weight, 13.33 mg/kg, 4.44 mg/kg, and 1.48 mg/kg), with 8 mice per group defined by dose, delivery method, and infecting strain. The 50% effective dose (ED50), i.e., the dose allowing survival of 50% of the infected mice, is calculated for each delivery route using probit analysis. | |
| Animal model | Male ICR mice (weight, 18 to 20 g) are inoculated intraperitoneally with 1 of 4 PRSP isolates (DR9, DR10, DR11, or DR14) suspended in 10% mucin, to induce a systemic S. pneumoniae infection. |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.0231 mL | 10.1155 mL | 20.2310 mL | 40.4621 mL |
| 5mM | 0.4046 mL | 2.0231 mL | 4.0462 mL | 8.0924 mL |
| 10mM | 0.2023 mL | 1.0116 mL | 2.0231 mL | 4.0462 mL |
| 20mM | 0.1012 mL | 0.5058 mL | 1.0116 mL | 2.0231 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.