This product is for research use only, not for human use. We do not sell to patients.
| Size | Price |
|---|---|
| 250mg | Get quote |
| 500mg | Get quote |
| 1g | Get quote |
Cat #: V4383 CAS #: 949142-50-1 Purity ≥ 99%
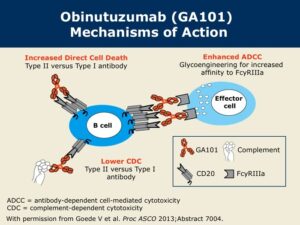
Description: Obinutuzumab (also called afutuzumab until 2009, originally GA101) is a novel glycoengineered and humanized Type II CD20 monoclonal antibody approved for treating non-Hodgkin lymphoma. Obinutuzumab is a humanized anti-CD20 monoclonal antibody, originated by GlycArt Biotechnology AG and developed by Roche as a cancer treatment. It was approved under the trade name Gazyva by the US FDA in 2013, and as Gazyvaro by the EMA in Europe, for the treatment of chronic lymphocytic leukemia in combination with chemotherapy in treatment-naive patients, and as a second line treatment for follicular lymphoma
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| CAS No. | 949142-50-1 |
|---|
| Protocol | In Vivo | Obinutuzumab is more active than rituximab administered at similar doses on established RL tumors. The antitumor effect of obinutuzumab against RL xenografts is dose dependent in terms of tumor growth inhibition (TGI). TGI is calculated using NCI formula at day 34 and shows values of 25, 75, and 85% for the 10, 30, and 100 mg/kg dosages of obinutuzumab, respectively. The higher doses of 30 and 100 mg/kg of obinutuzumab significantly inhibit the growth of RL tumors and result in some complete tumor remissions (10% and 30%, respectively). Tolerability of obinutuzumab with these regimens is excellent and no significant modification of body weight is observed. Obinutuzumab induces a strong antitumor effect, including complete tumor remission in the SU-DHL4 model and overall superior efficacy compared with both rituximab and ofatumumab. Obinutuzumab plus bendamustine achieves superior tumor growth inhibition versus rituximab plus bendamustine and shows a statistically significant effect versus the respective single treatments. Obinutuzumab plus chemotherapy is superior to the respective monotherapies. |
|---|
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































