This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 5g | $525 | Check With Us |
| 10g | $855 | Check With Us |
| 20g | $1280 | Check With Us |
Cat #: V2417 CAS #: 65473-14-5 Purity ≥ 98%
Description: Naftifine HCl (AW-105843; SN 105843) is an allylamine-based, synthetic, and broad spectrum antifungal drug for the topical treatment of tinea pedis, tinea cruris, and tinea corporis, probably involves selectively blocking sterol biosynthesis via inhibition of the squalene 2,3-epoxidase enzyme. Naftifine exhibits an interesting in vitro spectrum of activity against dermatophytes (38 strains; minimal inhibitory concentration (MIC) range 0.1 to 0.2 mg/mL), aspergilli (6 strains; MIC range, 0.8 to 12.5 mg/mL), Sporothrix schenckii (2 strains; MICs, 0.8 and 1.5 mg/mL), and yeasts of the genus Candida (77 strains; MIC range, 1.5 to greater than 100 mg/mL).
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 323.86 |
|---|---|
| Molecular Formula | C21H21N.HCl |
| CAS No. | 65473-14-5 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: <1 mg/mLr |
| Water: <1 mg/mLr | |
| Ethanol: 8 mg/mL (24.7 mM) | |
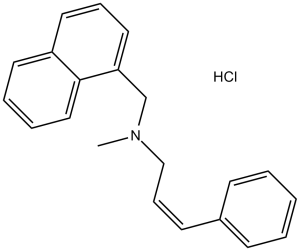
| SMILES Code | CN(CC1=C2C=CC=CC2=CC=C1)C/C=C/C3=CC=CC=C3.[H]Cl |
| Synonyms | SN 105843; SN105843; SN-105843; AW 105843; AW105843; AW-105843; AW 105-843; Naftifine hydrochloride; Naftin |
| Protocol | In Vitro | In vitro activity: Naftifine exhibits an interesting in vitro spectrum of activity against dermatophytes (38 strains; minimal inhibitory concentration (MIC) range 0.1 to 0.2 mg/mL), aspergilli (6 strains; MIC range, 0.8 to 12.5 mg/mL), Sporothrix schenckii (2 strains; MICs, 0.8 and 1.5 mg/mL), and yeasts of the genus Candida (77 strains; MIC range, 1.5 to greater than 100 mg/mL). The MIC of naftifine for C. albicans Δ63 is 100 mg/L in Sabouraud medium (initial pH 6.5). Naftifine (50 mg/L) gives greater than 99% inhibition of sterol biosynthesis both in whole cells and in cell extracts of C. albicans. The primary action of naftifine appears to be the blocking of fungal squalene epoxidation. |
|---|---|---|
| In Vivo | Naftifine HCl 2% cream results in clinical cure rate and clinical success rate of 33% and 84% after treatment for 4 weeks, and week 2 efficacy response rates in Naftifine HCl 2% subjects are all lower than at week 4 but are significantly higher than week 2 vehicle-treated counterparts. Naftifine causes interruption of fungal ergosterol synthesis and accumulation of squalene in fungal organisms. Naftifine also has demonstrated anti-inflammatory properties such as a reduction in superoxide production and a reduction in polymorphonuclear leukocyte chemotaxis/endothelial adhesion. Naftifine has shown good efficacy and safety for a variety of conditions and is a useful treatment that provides both antifungal action and relief of inflammatory signs and symptoms. Few adverse events have been noted with naftifine use, the most frequent being mild and transient burning, stinging, or itching in the application area. |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 3.0878 mL | 15.4388 mL | 30.8775 mL | 61.7551 mL |
| 5mM | 0.6176 mL | 3.0878 mL | 6.1755 mL | 12.3510 mL |
| 10mM | 0.3088 mL | 1.5439 mL | 3.0878 mL | 6.1755 mL |
| 20mM | 0.1544 mL | 0.7719 mL | 1.5439 mL | 3.0878 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































