MYCi975 (NUCC-0200975)
This product is for research use only, not for human use. We do not sell to patients.
For small sizes, please check our retail website as below: www.invivochem.com
| Size | Price | Stock |
|---|---|---|
| 500mg | $1700 | In Stock |
| 1g | $2500 | In Stock |
| 5g | $6750 | In Stock |
Cat #: V37600 CAS #: 2289691-01-4 Purity ≥ 98%
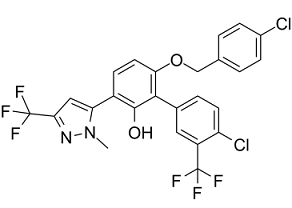
Description: MYCi975 (NUCC-0200975), an MYCi361 analog, is a novel, orally bioactive and potent MYC inhibitor (Kd = 3.2 μM) with potential anticancer activity.
Top Publications Citing Invivochem Products
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 561.31 |
|---|---|
| Molecular Formula | C25H16Cl2F6N2O2 |
| CAS No. | 2289691-01-4 |
| Protocol | In Vitro | MYCi975 (975) inhibited cell viability in a MYC-dependent manner (Figure 7A, S8A and S8B) and selectively suppressed E-box-luciferase activity (Figure 7B). To assess the molecular pathways modulated by MYCi treatment in an unbiased manner, RNA-seq experiments were performed using P493–6 and PC3 cells. The ability to repress MYC with tetracycline treatment in the P493–6 model allowed us to directly compare empirical MYC targets in these cells after turning MYC “off” to the genes regulated by 975 treatment. The results, shown in Figure 7C, indicate that 975 affected the expression of 3647 genes, the majority (69%) of which are MYC responsive. Among the 975-regulated genes that did not respond to MYC (and may therefore represent off-target effects), the top altered pathways were related to small molecule compound metabolism process, consistent with a general cellular response to exposure to organic small molecule (Figure 7D). Next, the effects of 975 were compared to those of 361 by RNA-seq in PC3 cells. 975 affected the expression of a smaller number of genes (n = 3095) compared to 361 (n = 5033), of which 66.4% were common between the two compounds (Figure 7E). GO biological process analysis of the common genes showed that cell cycle and DNA replication were among the top down regulated pathways, while pathways related to cell death, response to organic compound and ER stress were upregulated (Table S3). GSEA analysis of genes uniquely regulated by 361 (n = 2978) showed suppression of several sets that all share common leading edge genes encoding HIST1H proteins and the TCA cycle/respiratory electron transport (Table S4). However, no gene sets were significantly enriched in GSEA analysis of genes uniquely regulated by 975 in PC3 cells. These findings may partly explain the improved tolerability of 975 compared to 361 as will be shown below. |
|---|---|---|
| In Vivo | 975 exhibited excellent pharmacokinetic profiles following p.o., i.p. or i.v. administration (Figure S8C and S8D). The half-lives observed were 7 hr and 12 hr when dosed at 100 mg/kg and 250 mg/kg p.o. respectively. The Cmax values attained were 41533 ng/ml (74 μM) and 54000 ng/ml (96 μM) respectively. 975 significantly inhibited tumor growth (Figures 8A) and increased survival (Figures 8B) in the MycCaP allograft model with animals tolerating a 100 mg/kg/day i.p. dosing for 14 days. Analysis of tumor tissue showed increased pT58 and PD-L1 levels (Figure 8C) and enhanced tumor infiltration of CD3+ T cells (Figure 8D), B220+ B Cells (Figure 8E), and NKp46+ NK cells (Figure 8F) after 975 treatment. Therefore, the effect of combining 975 with anti-PD1 treatment was examined. 975 alone dosed at 100 mg/kg/day, 2 days on/2 days off slowed tumor growth, while the combination treatment with anti-PD1 (100 μg/day, on alternating 2 days on/2 days off) resulted in a synergistic suppression of tumor growth (Figure 8G and S8E). Similar to 361, 975 treatment inhibited MycCaP tumors grown in immunocompetent FVB mice more strongly than in immunodeficient NSG mice (Figure 8H), indicating that full anti-tumor efficacy of 975 is also dependent on an intact immune system. Treatment of Lewis Lung Carcinoma (LLC1)-bearing mice with 975 (100 mg/kg/day) inhibited tumor growth with no changes in body weight (Figure 8I and S8F). NSG mice bearing MV-411 AML xenografts were treated with 975 (50 mg/kg/day) or Ara-C (20 mg/kg/day) 5 days a week. In this model, the lower dose of 975 and the immunodeficient host background may explain reduced efficacy as a single agent. 975 synergized with Ara-C with no obvious impact on mouse body weight (Figure 8J and S8G). |
These protocols are for reference only. InvivoChem does not
independently validate these methods.
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.7815 mL | 8.9077 mL | 17.8155 mL | 35.6309 mL |
| 5mM | 0.3563 mL | 1.7815 mL | 3.5631 mL | 7.1262 mL |
| 10mM | 0.1782 mL | 0.8908 mL | 1.7815 mL | 3.5631 mL |
| 20mM | 0.0891 mL | 0.4454 mL | 0.8908 mL | 1.7815 mL |
The molarity calculator equation
Mass(g) = Concentration(mol/L) × Volume(L) × Molecular Weight(g/mol)
Mass
=
Concentration
×
Volume
×
Molecular Weight*
The dilution calculator equation
Concentration(start)
×
Volume(start)
=
Concentration(final)
×
Volume(final)
This equation is commonly abbreviated as: C1 V1 = C2 V2
Concentration(start)
C1
×
Volume(start)
V1
=
Concentration(final)
C2
×
Volume(final)
V2
Step One: Enter information below
Dosage mg/kg
Average weight of animals g
Dosing volume per animal µL
Number of animals
Step Two: Enter the in vivo formulation
%DMSO
+
%
+
%Tween 80
+
%ddH2O
Calculation Results:
Working concentration:
mg/ml;
Method for preparing DMSO master liquid:
mg
drug pre-dissolved in
µL
DMSO(Master liquid concentration
mg/mL)
,Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation:
Take
µL
DMSO master liquid, next add
µL
PEG300, mix and clarify, next add
µL
Tween 80,mix and clarify, next add
µL
ddH2O,mix and clarify.
Note:
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































