This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 500mg | $249 | Check With Us |
| 1g | $390 | Check With Us |
| 5g | $975 | Check With Us |
Cat #: V2218 CAS #: 96036-03-2 Purity ≥ 98%
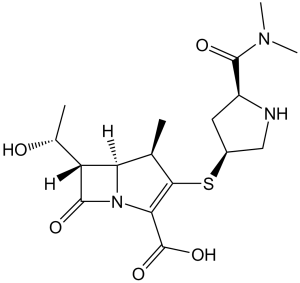
Description: Meropenem (formerly known as SM-7338; SM7338; ICI194660; Vabomere; Merrem), a beta-lactam of the carbapenem class, is an ultra-broad-spectrum injectable β-lactam antibiotic used to treat a wide variety of infections. Meropenem has been shown to inhibit penicillinase-negative, -positive and methicillin-susceptible staphylococci. Meropenem has an antibacterial spectrum which is broadly similar to that of imipenem but, whilst slightly less active against staphylococci and enterococci, it is more active against Pseudomonas aeruginosa, all Enterobacteriaceae and Haemophilus influenzae. Meropenem demonstrates antagonism with several other beta-lactams against strains producing Type I cephalosporinases.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 383.46 |
|---|---|
| Molecular Formula | C17H25N3O5S |
| CAS No. | 96036-03-2 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 76 mg/mL (198.2 mM)r |
| Water: 8 mg/mL (20.9 mM)r | |
| Ethanol: <1 mg/mL | |
| SMILES Code | O=C(C(N12)=C(S[C@@H]3CN[C@H](C(N(C)C)=O)C3)[C@H](C)[C@]2([H])[C@@H]([C@H](O)C)C1=O)O |
| Synonyms | SM-7338; ICI-194660; SM 7338; ICI 194660; SM7338; ICI194660; Vabomere. |
| Protocol | In Vitro | In vitro activity: Meropenem has an antibacterial spectrum which is broadly similar to that of imipenem but, whilst slightly less active against staphylococci and enterococci, it is more active against Pseudomonas aeruginosa, all Enterobacteriaceae and Haemophilus influenzae. Meropenem is two- to four-fold more active than imipenem against Gram-negative organisms and its spectrum of antimicrobial activity is wider than those of all other drugs tested. Meropenem MICs are not significantly influenced by high inocula and the drug is generally bactericidal. Meropenem demonstrates antagonism with several other beta-lactams against strains producing Type I cephalosporinases. Meropenem binds most strongly to penicillin-binding protein 2 of Escherichia coli and Pseudomonas aeruginosa, and to penicillin-binding proteins 1 of Staphylococcus aureus. Meropenem is a new carbapenem antibiotic which differs chemically from imipenem/cilastatin by having a 1-beta-methyl substitution, providing it with excellent intrinsic stability to human renal dehydropeptidase-I. Meropenem has one identified metabolite, a beta-lactam ring-opened form which is devoid of microbiological activity. Kinase Assay: Meropenem (SM 7338), a new parenteral carbapenem demonstrated increased activity as compared to imipenem against 336 strains of Neisseria gonorrhoeae, 119 strains of Haemophilus influenzae, and 110 strains of H. Ceftriaxone and ciprofloxacin demonstrated activity superior to that of both carbapenems while the activity of ceftazidime was similar to that of Meropenem (SM 7338). Cell Assay: The meropenem MICs for penicillin-resistant Streptococcus pneumoniae were higher than for the penicillin-susceptible strains but the organisms remained susceptible. Clinical susceptibility in vitro to meropenem was defined by MICs of ≤ 4 mg/L, intermediate susceptibility by MICs of 8 mg/L and MICs of ≥ 16 mg/L define resistance; equivalent figures for zones of growth inhibition were ≥ 14 (susceptible), 12-13 (intermediate) and ≤ 11 (resistant) mm[1].Meropenem was 2- to 4-fold more active than imipenem against Gram-negative organisms and its spectrum of antimicrobial activity was wider than those of all other drugs tested.Meropenem inhibited all anaerobic bacteria at less than or equal to 8 mg/l and 0.25 mg/l inhibited 50% of strains. Meropenem MICs were not significantly influenced by high inocula and the drug was generally bactericidal.Meropenem bound most strongly to penicillin-binding protein 2 of Escherichia coli and Pseudomonas aeruginosa, and to penicillin-binding proteins 1 of Staphylococcus aureus. Meropenem had one identified metabolite, a β-lactam ring-opened form which is devoid of microbiological activity. |
|---|---|---|
| In Vivo | Meropenem significantly increases the plamsa total clearance of valproate to about 1.5 times the control (6.09 mL/min/kg vs. 4.28 mL/min/kg) in rabbits. Meropenem significantly increases the urinary excretion of valproate- glucuronide in rabbits. | |
| Animal model | In rabbits, meropenem significantly increased the plamsa total clearance of valproate to about 1.5 times compared to the control (6.09 mL/min/kg vs. 4.28 mL/min/kg). Meropenem significantly increased the urinary excretion of valproate- glucuronide in rabbits. | |
| Dosages | 60 mg/kg | |
| Administration | Intraperitoneal injection; once |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.6078 mL | 13.0392 mL | 26.0783 mL | 52.1567 mL |
| 5mM | 0.5216 mL | 2.6078 mL | 5.2157 mL | 10.4313 mL |
| 10mM | 0.2608 mL | 1.3039 mL | 2.6078 mL | 5.2157 mL |
| 20mM | 0.1304 mL | 0.6520 mL | 1.3039 mL | 2.6078 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































