This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 250mg | $450 | Check With Us |
| 500mg | $750 | Check With Us |
| 1g | $1125 | Check With Us |
Cat #: V2352 CAS #: 88678-31-3 Purity ≥ 98%
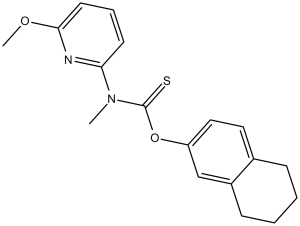
Description: Liranaftate (formerly M-732; Piritetrate; Zefnart) is a thiocarbamate-based inhibitor of squalene epoxidase with fungicidal activities. It inhibits fungal squalene epoxidase, which is an enzyme that plays a key role in the synthesis of sterol, compouds essential for cell membrane integrity. By preventing ergosterol synthesis and causing accumulation of squalene, this agent increases cell membrane permeability, cell leakage and eventually cell lysis. Liranaftate showed excellent fungistatic activity against the conidia of T. rubrum. For each of these agents, the MIC after 14 days of contact was 0.009 g/ml. The liranaftate-induced decrease in the MCC occurred from 9 days onwards; MCC at 14 days was 0.039 g/ml. In time-kill studies, liranaftate showed the greatest decrease to a below detection limit in viable counts of T rubrum. The degree of killing of the strain by amorolfine was not greater than that seen by liranaftate, and little reduction of the viable counts by luliconazole and ketoconazole was observed irrespective of concentrations of the agents.
References: [1]. Oku, Y., et al., [Fungicidal activity of liranaftate against Trichophyton rubrum]. Nihon Ishinkin Gakkai Zasshi, 2002. 43(3): p. 181-7.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 328.43 |
|---|---|
| Molecular Formula | C18H20N2O2S |
| CAS No. | 88678-31-3 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: <1 mg/mLr |
| Water: <1 mg/mLr | |
| Ethanol: <1 mg/mL | |
| Solubility In Vivo | Chemical Name: O-(5,6,7,8,-Tetrahydro-2-naphthyl) 6-methoxy-N-methylthio-2-pyridinecarbamate InChi Key: GTLOCRWOJSGVDM-UHFFFAOYSA-N InChi Code: InChI=1S/C18H20N2O3S/c1-22-17-9-5-8-16(19-17)20(24-2)18(21)23-15-11-10-13-6-3-4-7-14(13)12-15/h5,8-12H,3-4,6-7H2,1-2H3 SMILES Code: O=C(OC1=CC=C2CCCCC2=C1)N(SC)C3=NC(OC)=CC=C3 |
| SMILES Code | O=C(OC1=CC=C2CCCCC2=C1)N(SC)C3=NC(OC)=CC=C3 |
| Synonyms | M732; M 732; M-732; Piritetrate; Zefnart |
| Protocol | In Vitro | Liranaftate showed excellent fungistatic activity against the conidia of T. rubrum. For each of these agents, the MIC after 14 days of contact was 0.009 g/ml. The liranaftate-induced decrease in the MCC occurred from 9 days onwards; MCC at 14 days was 0.039 g/ml . In time-kill studies, liranaftate showed the greatest decrease to a below detection limit in viable counts of T rubrum. The degree of killing of the strain by amorolfine was not greater than that seen by liranaftate, and little reduction of the viable counts by luliconazole and ketoconazole was observed irrespective of concentrations of the agents . |
|---|
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 3.0448 mL | 15.2239 mL | 30.4479 mL | 60.8958 mL |
| 5mM | 0.6090 mL | 3.0448 mL | 6.0896 mL | 12.1792 mL |
| 10mM | 0.3045 mL | 1.5224 mL | 3.0448 mL | 6.0896 mL |
| 20mM | 0.1522 mL | 0.7612 mL | 1.5224 mL | 3.0448 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































