This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 5mg | $345 | To Be Confirmed |
| 10mg | $500 | To Be Confirmed |
| 25mg | $860 | To Be Confirmed |
| 50mg | $1260 | To Be Confirmed |
| 100mg | $1800 | To Be Confirmed |
| 250mg | $2980 | To Be Confirmed |
Cat #: V3774 CAS #: 1207989-22-7 Purity ≥ 98%
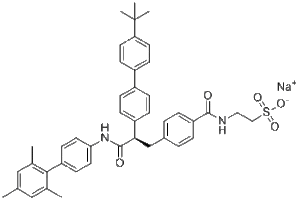
Description: LGD-6972 soidum (LGD6972; MB-11262; MB11262) is a novel and orally bioavailable glucagon receptor antagonist with antidiabetic effects. It has linear plasma pharmacokinetics consistent with once daily dosing that is comparable in healthy and T2DM subjects. It also reduces plasma glucose in the postprandial state. Dose-dependent increases in fasting plasma glucagon are observed, but glucagon levels decrease and insulin levels increase after an oral glucose load in T2DM subjects. Inhibition of glucagon action by LGD-6972 was associated with decreases in glucose in both healthy and T2DM subjects, the magnitude of which was sufficient to predict improvement in glycaemic control with longer treatment duration in T2DM patients. The safety and pharmacological profile of LGD-6972 after 14 days of dosing supports continued clinical development.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 724.89 |
|---|---|
| Molecular Formula | C43H45N2NaO5S |
| CAS No. | 1207989-22-7 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: ≥ 31 mg/mL |
| Water: N/A | |
| Ethanol: N/A | |
| Synonyms | LGD6972; LGD 6972; LGD-6972 sodium |
| Protocol | In Vitro | In vitro activity: LGD-6972 is a novel and orally available glucagon receptor antagonist that has linear plasma pharmacokinetics consistent with once daily dosing that is comparable in healthy and T2DM subjects. It also reduces plasma glucose in the postprandial state. Dose-dependent increases in fasting plasma glucagon are observed, but glucagon levels decrease and insulin levels increase after an oral glucose load in T2DM subjects. Inhibition of glucagon action by LGD-6972 was associated with decreases in glucose in both healthy and T2DM subjects, the magnitude of which was sufficient to predict improvement in glycaemic control with longer treatment duration in T2DM patients. The safety and pharmacological profile of LGD-6972 after 14 days of dosing supports continued clinical development. |
|---|---|---|
| In Vivo | LGD-6972 reduces plasma glucose in the postprandial state. Dose-dependent increases in fasting plasma glucagon are observed, but glucagon levels decrease and insulin levels increase after an oral glucose load in T2DM subjects. LGD-6972 is well tolerated at the doses tested without dose-related or clinically meaningful changes in clinical laboratory parameters. No subject experiences hypoglycaemia. |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.3795 mL | 6.8976 mL | 13.7952 mL | 27.5904 mL |
| 5mM | 0.2759 mL | 1.3795 mL | 2.7590 mL | 5.5181 mL |
| 10mM | 0.1380 mL | 0.6898 mL | 1.3795 mL | 2.7590 mL |
| 20mM | 0.0690 mL | 0.3449 mL | 0.6898 mL | 1.3795 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































