This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 250mg | $265 | Check With Us |
| 500mg | $445 | Check With Us |
| 1g | $660 | Check With Us |
Cat #: V1877 CAS #: 201530-41-8 Purity ≥ 98%
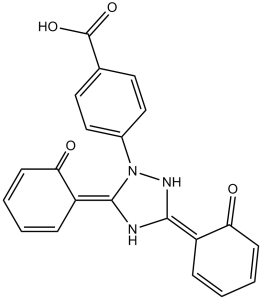
Description: Deferasirox (also known as CGP-72670, ICL-670) is an orally bioavailable iron chelator used for the treatment of iron-overload disease. In DMS-53 lung carcinoma and SK-N-MC neuroepithelioma cell lines, deferasirox inhibited cells proliferation. Deferasirox inhibited iron uptake from human transferrin and mobilized cellular 59Fe. In two oesophageal adenocarcinoma cell lines OE33 and OE19, deferasirox with a standard chemotherapeutic agent inhibited cellular viability and proliferation. Deferasirox effectively chelates iron from Rhizopus oryzae and demonstrates cidal activity in vitro against 28 of 29 clinical isolates of Mucorales at concentrations well below clinically achievable serum levels.
References: [1]. Sobbe A, et al. Inconsistent hepatic antifibrotic effects with the iron chelator deferasirox. J Gastroenterol Hepatol. 2015 Mar;30(3):638-45.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 373.36 |
|---|---|
| Molecular Formula | C21H15N3O4 |
| CAS No. | 201530-41-8 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 74 mg/mL (198.2 mM)r |
| Water: <1 mg/mLr | |
| Ethanol: 2 mg/mL (5.35 mM) | |
| Solubility In Vivo | 30% PEG400+0.5% Tween80+5% Propylene glycol: 30 mg/mL |
| SMILES Code | O=C(O)C1=CC=C(N2N=C(C3=CC=CC=C3O)N=C2C4=CC=CC=C4O)C=C1 |
| Synonyms | CGP-72670; CGP 72670; CGP72670; ICL-670; ICL670; ICL 670; ICL-670A; ICL670A; IC L670A; Deferasirox; Exjade; Desirox; Defrijet; Desifer. |
| Protocol | In Vitro | In vitro activity: Deferasirox effectively chelates iron from Rhizopus oryzae and demonstrates cidal activity in vitro against 28 of 29 clinical isolates of Mucorales at concentrations well below clinically achievable serum levels. Deferasirox incubation induces a significant inhibition of NF-κB activity and a cytoplasmic sequestration of its active subunit p65 in an inactive form in 28 of 40 peripheral blood samples. Deferasirox inhibits three human myeloid cell lines (K562, U937, and HL60) with IC50 of 17-50 mM. Deferasirox is cidal in vitro against A. fumigatus, with an MIC and MFC of 25 and 50 mg/L, respectively. Cell Assay: In DMS-53 lung carcinoma and SK-N-MC neuroepithelioma cell lines, deferasirox inhibited cells proliferation. Deferasirox inhibited iron uptake from human transferrin and mobilized cellular 59Fe. In two oesophageal adenocarcinoma cell lines OE33 and OE19, deferasirox with a standard chemotherapeutic agent inhibited cellular viability and proliferation. |
|---|---|---|
| In Vivo | Deferasirox significantly improves survival and decreased tissue fungal burden in diabetic ketoacidotic or neutropenic mice with mucormycosis, with an efficacy similar to that of liposomal amphotericin B. Deferasirox treatment also enhances the host inflammatory response to mucormycosis. Deferasirox synergistically improves survival and reduces tissue fungal burden when combined with liposomal amphotericin B. Deferasirox administered p.o. to rats is absorbed to at least 75%, and the bioavailability is 26%.Deferasirox is present in the blood circulation mainly in the unchanged form and as its iron complex, Fe(deferasirox)2, after intravenous and p.o. administration. Deferasirox is 99.2% bound to plasma proteins. Deferasirox monotherapy modestly prolongs survival of mice with IPA. | |
| Animal model | In nude mice bearing DMS-53 lung carcinoma xenografts, deferasirox inhibited tumor growth. Also, deferasirox increased cleaved caspase-3, cleaved poly(ADP-ribose) polymerase 1, the cyclin-dependent kinase inhibitor p21CIP1/WAF1 and the expression of the metastasis suppressor protein N-myc downstream-regulated gene 1 while reducing cyclin D1, which suggested deferasirox is an effective antitumor agent. In human xenograft models, deferasirox significantly inhibited tumour growth, which was associated with the decrease in iron levels. |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.6784 mL | 13.3919 mL | 26.7838 mL | 53.5676 mL |
| 5mM | 0.5357 mL | 2.6784 mL | 5.3568 mL | 10.7135 mL |
| 10mM | 0.2678 mL | 1.3392 mL | 2.6784 mL | 5.3568 mL |
| 20mM | 0.1339 mL | 0.6696 mL | 1.3392 mL | 2.6784 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































