This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 2g | $170 | Check With Us |
| 10g | $520 | Check With Us |
| 20g | $780 | Check With Us |
Cat #: V2040 CAS #: 91832-40-5 Purity ≥ 98%
Description: Cefdinir (also known as FK 482, PD 134393, CI-983) is a potent, orally bioavailable, semi-synthetic, and broad-spectrum cephalosporin class of antibiotic used to treat bacterial infections in many different parts of the body including the ear, sinus, throat, and skin. . Cefdinir, a new oral 2-amino-5-thiazolyl cephalosporin, inhibits the luminol-amplified chemiluminescence (LACL) response of human neutrophils stimulated by PMA but not opsonized zymosan, in a concentration-dependent but not time-dependent manner. Cefdinir inhibits LACL generation in cell-free systems consisting of H2O2, NaI, and either horseradish peroxidase or amyeloperoxidase-containing neutrophil extract. Cefdinir impairs LACL response induced by the calcium ionophore A23187 and FMLP, and this impairment is increased in cytochalasin B-treated neutrophils.
References: [1]. Soejima R. Cefdinir. Jpn J Antibiot. 1992 Oct;45(10):1239-52.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 395.41 |
|---|---|
| Molecular Formula | C14H13N5O5S2 |
| CAS No. | 91832-40-5 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 79 mg/mL (199.8 mM)r |
| Water: <1 mg/mLr | |
| Ethanol: <1 mg/mL | |
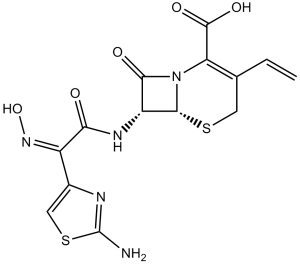
| SMILES Code | C=CC1=C(N2[C@@H]([C@@H](C2=O)NC(=O)/C(=N\O)/C3=CSC(=N3)N)SC1)C(=O)O |
| Synonyms | FK 482, PD 134393, CI-983; PD-134393; PD134393; Omnicef; CFDN; Cefdinirum; Cefdinyl; CI 983; CI983; FK482; FK-482 |
| Protocol | In Vitro | In vitro activity: Cefdinir, a new oral 2-amino-5-thiazolyl cephalosporin, inhibits the luminol-amplified chemiluminescence (LACL) response of human neutrophils stimulated by PMA but not opsonized zymosan, in a concentration-dependent but not time-dependent manner. Cefdinir inhibits LACL generation in cell-free systems consisting of H2O2, NaI, and either horseradish peroxidase or amyeloperoxidase-containing neutrophil extract. Cefdinir impairs LACL response induced by the calcium ionophore A23187 and FMLP, and this impairment is increased in cytochalasin B-treated neutrophils. Cefdinir directly inhibits the activity of myeloperoxidase-containing neutrophil extract released into the extracellular medium during neutrophil stimulation by soluble mediators, but has no effect on that released into the phagolysosome during phagocytosis. Cefdinir demonstrates excellent activity against a wide range of gram-positive and gram-negative bacteria. Cefdinir is resistant to a broad variety of β-lactamases and exhibits a β-lactam stability profile generally better than those observed with cefaclor and cefuroxime. Cefdinir elimination is primarily mediated by the kidney. Cefdinir interacts with the dipeptide transporters PEPT1 and PEPT2. Cefdinir tubular reabsorption is substantial, that Cefdinir tubular secretion is inhibitable by probenecid, and that this secretion is probably mediated by the renal organic anion secretory pathway. |
|---|
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.5290 mL | 12.6451 mL | 25.2902 mL | 50.5804 mL |
| 5mM | 0.5058 mL | 2.5290 mL | 5.0580 mL | 10.1161 mL |
| 10mM | 0.2529 mL | 1.2645 mL | 2.5290 mL | 5.0580 mL |
| 20mM | 0.1265 mL | 0.6323 mL | 1.2645 mL | 2.5290 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































