This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 5mg | $280 | To Be Confirmed |
| 10mg | $410 | To Be Confirmed |
| 25mg | $720 | To Be Confirmed |
| 50mg | $1060 | To Be Confirmed |
| 100mg | $1530 | To Be Confirmed |
| 250mg | $2590 | To Be Confirmed |
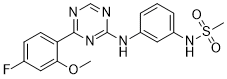
Cat #: V3937 CAS #: 1335490-39-5 Purity ≥ 98%
Description: BAY-958 is a lead compound for Atuveciclib (formerly known as BAY-1143572) which is novel, potent, oral and highly selective PTEFb/CDK9 inhibitor. It inhibits CDK9/CycT1 with an IC50 of 13 nM and is more than 100-fold more selective for CDK9 over CDK2. It also inhibits GSK3 kinase with IC50 values of 45 nM and 87 nM for GSK3α and GSK3β respectively. Atuveciclib is currently in Phase I clinical trial. Selective inhibition of exclusively transcription-regulating PTEFb/CDK9 is a promising new approach in cancer therapy. Starting from lead compound BAY-958, lead optimization efforts strictly focusing on kinase selectivity, physicochemical and DMPK properties finally led to the identification of the orally available clinical candidate atuveciclib (BAY 1143572). Structurally characterized by an unusual benzyl sulfoximine group, BAY 1143572 exhibited the best overall profile in vitro and in vivo, including high efficacy and good tolerability in xenograft models in mice and rats. BAY 1143572 is the first potent and highly selective PTEFb/CDK9 inhibitor to enter clinical trials for the treatment of cancer.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 389.4 |
|---|---|
| Molecular Formula | C17H16FN5O3S |
| CAS No. | 1335490-39-5 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: >10mM |
| Water: <1mg/mL | |
| Ethanol:<1mg/mL | |
| Synonyms | BAY-958; BAY 958; BAY958 |
| Protocol | In Vitro | In vitro activity: BAY-958 is a lead compound for Atuveciclib (formerly known as BAY-1143572) which is novel, potent, oral and highly selective PTEFb/CDK9 inhibitor. It inhibits CDK9/CycT1 with an IC50 of 13 nM and is more than 100-fold more selective for CDK9 over CDK2. It also inhibits GSK3 kinase with IC50 values of 45 nM and 87 nM for GSK3α and GSK3β respectively. Atuveciclib is currently in Phase I clinical trial. Selective inhibition of exclusively transcription-regulating PTEFb/CDK9 is a promising new approach in cancer therapy. Starting from lead compound BAY-958, lead optimization efforts strictly focusing on kinase selectivity, physicochemical and DMPK properties finally led to the identification of the orally available clinical candidate atuveciclib (BAY 1143572). Structurally characterized by an unusual benzyl sulfoximine group, BAY 1143572 exhibited the best overall profile in vitro and in vivo, including high efficacy and good tolerability in xenograft models in mice and rats. BAY 1143572 is the first potent and highly selective PTEFb/CDK9 inhibitor to enter clinical trials for the treatment of cancer. Kinase Assay: Merck Millipore CDK assays: Assays were performed according to the Merck Millipore KinaseProfilerTM standard protocols, with an ATP concentration of 10 μm.. Cell Assay: HeLa human cervical tumor cells (CCL‐2) were obtained from the American Type Culture Collection (Manassas, USA) and MOLM‐13 human acute myeloid leukemia cells (ACC 554) were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Authentication of cell lines was conducted at the German Collection of Microorganisms and Cell Cultures via PCR‐based DNA profiling of polymorphic short tandem repeats. Cells were propagated under the suggested growth conditions in a humidified 37 °C incubator. Proliferation assays were conducted in 96‐well plates at densities of 3000 (HeLa) and 5000 (MOLM‐13) cells per well in the growth medium containing 10 % fetal calf serum (FCS). Cells were treated in quadruplicate with serial dilutions of test compounds for 96 h. Relative cell numbers were quantified by crystal violet staining (HeLa) or CellTitre‐Glo Luminescent Cell Viability Assay (Promega) (MOLM‐13). IC50 values (inhibitory concentration at 50 % of maximal effect) were determined by means of a four‐parameter fit on measurement data which were normalized to vehicle (DMSO) treated cells (=100 %) and measurement readings taken immediately before compound exposure (=0 %). |
|---|---|---|
| In Vivo | In an in vivo pharmacokinetic study in rats, BAY 1143572 showed low blood clearance (CLb 1.1 L/h/kg). The volumes of distribution (V ss) of BAY 1143572 is 1.0 L/kg. BAY 1143572 shows significantly improved oral bioavailability of 54 %. The blood/plasma ratios is about 1. It does not show significant inhibition of cytochrome P450 activity, with IC50 values >20 μM. The administration of BAY 1143572 in immunocompromized NOD/Shi-scid/IL-2Rγ null (NOG) mice xenografted with patient-derived ATL cells greatly reduced the infiltration of ATL cells into organs, such as liver and bone marrow. Decreased human soluble IL2R levels in serum were also observed, which indicated a reduction of ATL tumor burden. | |
| Animal model | Immunocompromized NOD/Shi-scid/IL-2Rγ null (NOG) mice xenografted with patient-derived ATL cells and in vivo pharmacokinetic in rats |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.5681 mL | 12.8403 mL | 25.6805 mL | 51.3611 mL |
| 5mM | 0.5136 mL | 2.5681 mL | 5.1361 mL | 10.2722 mL |
| 10mM | 0.2568 mL | 1.2840 mL | 2.5681 mL | 5.1361 mL |
| 20mM | 0.1284 mL | 0.6420 mL | 1.2840 mL | 2.5681 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































