This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 5mg | $116 | 3-6 Days |
| 10mg | $180 | 3-6 Days |
| 25mg | $279 | 3-6 Days |
| 50mg | $450 | 3-6 Days |
| 100mg | $750 | 3-6 Days |
| 250mg | $1250 | 3-6 Days |
| 500mg | $1950 | 3-6 Days |
| 1g | $2750 | 3-6 Days |
Cat #: V4243 CAS #: 1173900-37-2 Purity ≥ 98%
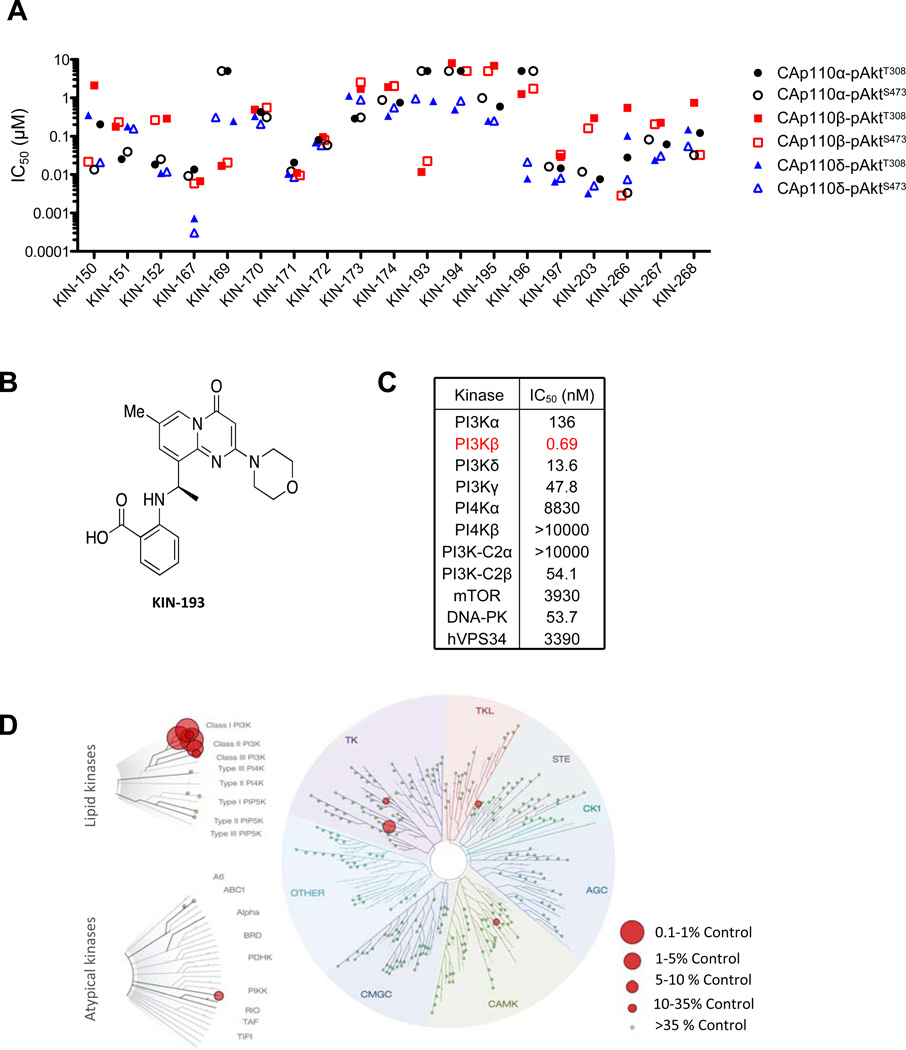
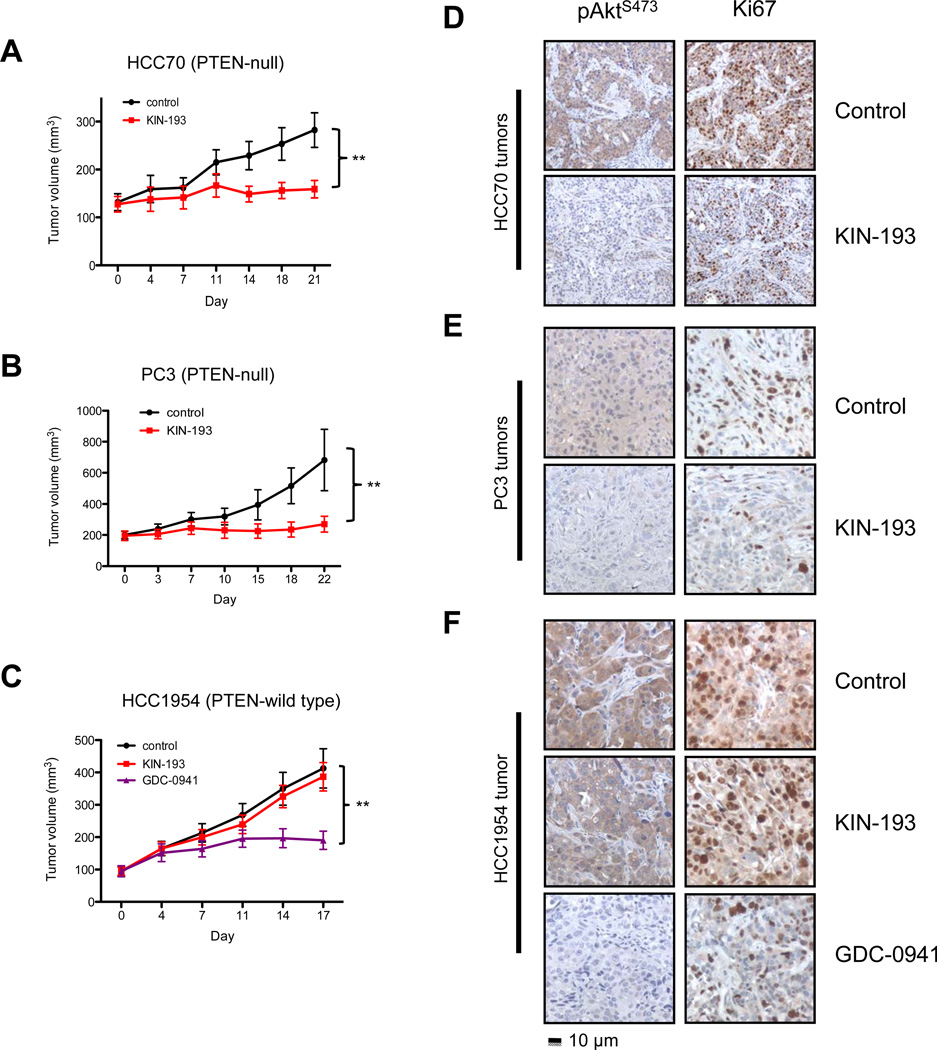
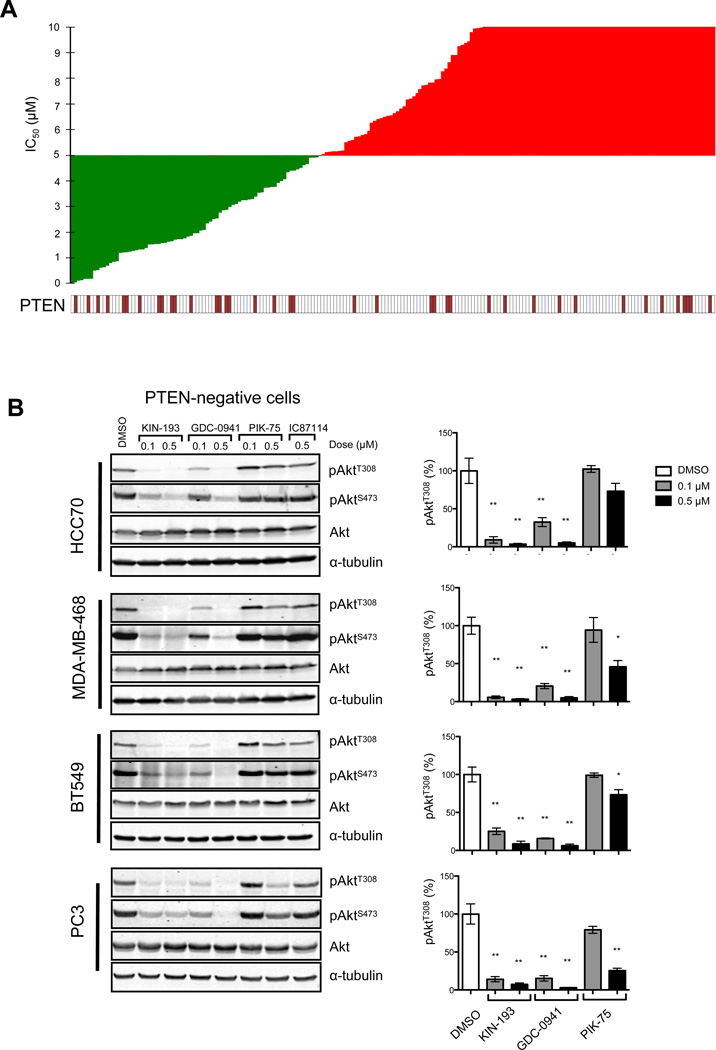
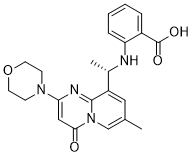
Description: AZD6482 S-isomer, is an isomer of AZD6482 which is a novel, potent, selective and ATP competitive PI3Kβ inhibitor with IC50 of 10 nM, 8-, 87- and is 109-fold more selective to PI3Kβ than PI3Kδ, PI3Kα and PI3Kγ in cell-free assays. Genetic approaches have shown that the p110β isoform of class Ia phosphatidylinositol-3-kinase (PI3K) is essential for the growth of PTEN-null tumors. Thus, it is desirable to develop p110β-specific inhibitors for cancer therapy. In vitro, AZD6482 inhibited insulin-activated uptake of human adpocyte glucose (IC50=4.4 μm). A full anti-thrombotic response with no increased bleeding or blood loss was observed in dog treated with AZD6482 in vivo. It also showed good tolerance in a three-hour AZD6482 infusion in healthy volunteers. There was an approx.10-20% rise at 5.3 μm (highest plasma concentration) according to the homeostasis model analysis index.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 408.45 |
|---|---|
| Molecular Formula | C22H24N4O4 |
| CAS No. | 1173900-37-2 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 82 mg/mL (200.75 mM) |
| Water:<1 mg/mL | |
| Ethanol: 10 mg/mL (24.48 mM) | |
| Solubility In Vivo | 30% PEG400+0.5% Tween80+5% Propylene glycol: 30 mg/mL |
| Protocol | In Vitro | Kinase Assay: The inhibition of PI3Kβ, PI3Kα, PI3Kγ, and PI3Kδ is evaluated in an AlphaScreen based enzyme activity assay using human recombinant enzymes. The assay measures PI3K-mediated conversion of PIP2 to PIP3. Biotinylated PIP3, a GST-tagged pleckstrin homology (PH) domain and the two AlphaScreen beads form a complex that elicits a signal upon laser excitation at 680 nm. The PIP3 formed in the enzyme reaction competes with the biotinylated PIP3 for binding to the PH domain thus reducing the signal with increasing enzyme product. The AZD6482 is dissolved in DMSO and added to 384 well plates. PBKβ, PBKα, PBKγ, or PBKδ is added in a Tris buffer (50 mM Tris pH 7.6, 0.05% CHAPS, 5 mM DTT, and 24 mM MgCl2) and allowed to preincubate with AZD6482 for 20 minutes prior to the addition of substrate solution containing PIP2 and ATP. The enzyme reaction is stopped after 20 minutes by addition of stop solution containing EDTA and biotin-PIP3, followed by addition of detection solution containing GST-grpl PH and AlphaScreen beads. Plates are left for a minimum of 5 hours in the dark prior to analysis. The final concentration of DMSO, ATP and PIP2 in the assay are, 0.8%, 4 μM, and 40 μM, respectively. IC50 values are calculated according to the equation, y = {a+[(b-a)/(l+(x/IC50)s)]}, where y = % inhibition; a = 0%; b = 100%; s = the slope of the concentration-response curve; x = AZD6482 concentration. Cell Assay: BT20, U87MG, and SKOV3 cells are plated at 3,000 cell/well in 96-well culture plates in growth medium with 10% FBS. Cells are incubated overnight and treated with DMSO (0.1% final) or serial diluted compound for 3 days. Resazurin is added to 0.1 mg/mL. Plates are incubated at 37 °C in 5% CO2 for 3 hours. Fluorescence signals are read as emission at 590 nm after excitation at 530 nm. IC50 values are calculated by plotting fluorescence intensity to drug concentration in nonlinear curve. For assay of washed platelet aggregation (WPA), the platelet pellets are isolated from human blood and re-suspended to 2 × 1015/L in Tyrodes buffer (TB) containing 1 μM hirudin and 0.02 U/mL apyrase. Then, the platelet suspension is left to rest at room temperature for 30 min. Just prior to time for assay, CaCl2 is added to a final concentration of 2 mM. AZD6482, dissolved in DMSO, is added to a 96-well plate prior to the addition of the washed platelet suspension. The platelet suspension is preincubated with AZD6482 for 5 min. Light absorption at 650 nm is recorded before and after a 5 min plate shake and referred to as recording 0 (R0) and Rl. A mouse anti-human CD9 antibody is added (at a donor specific concentration) to each well prior to next 10 min plate shake and light absorption recording; R2. For data analysis, light absorbance in wells with TB are subtracted from all readings before percent aggregation is calculated according the formula: [(R1-R2)/R1] × 100 = % aggregation. Spontaneous aggregation or pro-aggregatory effect of the inhibitor is evaluated by the same formula, [(R0-Rl)/R0] × 100 = % aggregation. IC50 values are calculated according to the equation, y = {a+[(b-a)/(l+(x/IC50)s)]}, where y = % inhibition; a = 0%; b = 100%; s = the slope of the concentration-response curve; x = AZD6482 concentration. A ZD6482 also inhibits PI3Kα, γ, and δ, with IC50 of 80 nM to 1.09 μM, which are significantly lower than its (+)-enantiomer (S-form). AZD6482 is an antiplatelet agent; it blocks platelet activation adhesion/aggregation and promotes platelet disaggregation in assay of washed platelet aggregation (WPA), with an IC50 value of 6 nM. Furthermore, by targeting PI3Kβ, AZD6482 specifically inhibits thrombosis without interfering with normal haemostasis. Therefore, AZD6482 is used as an anti-thrombotic drug for the prophylaxis of thrombotic disorders. |
|---|---|---|
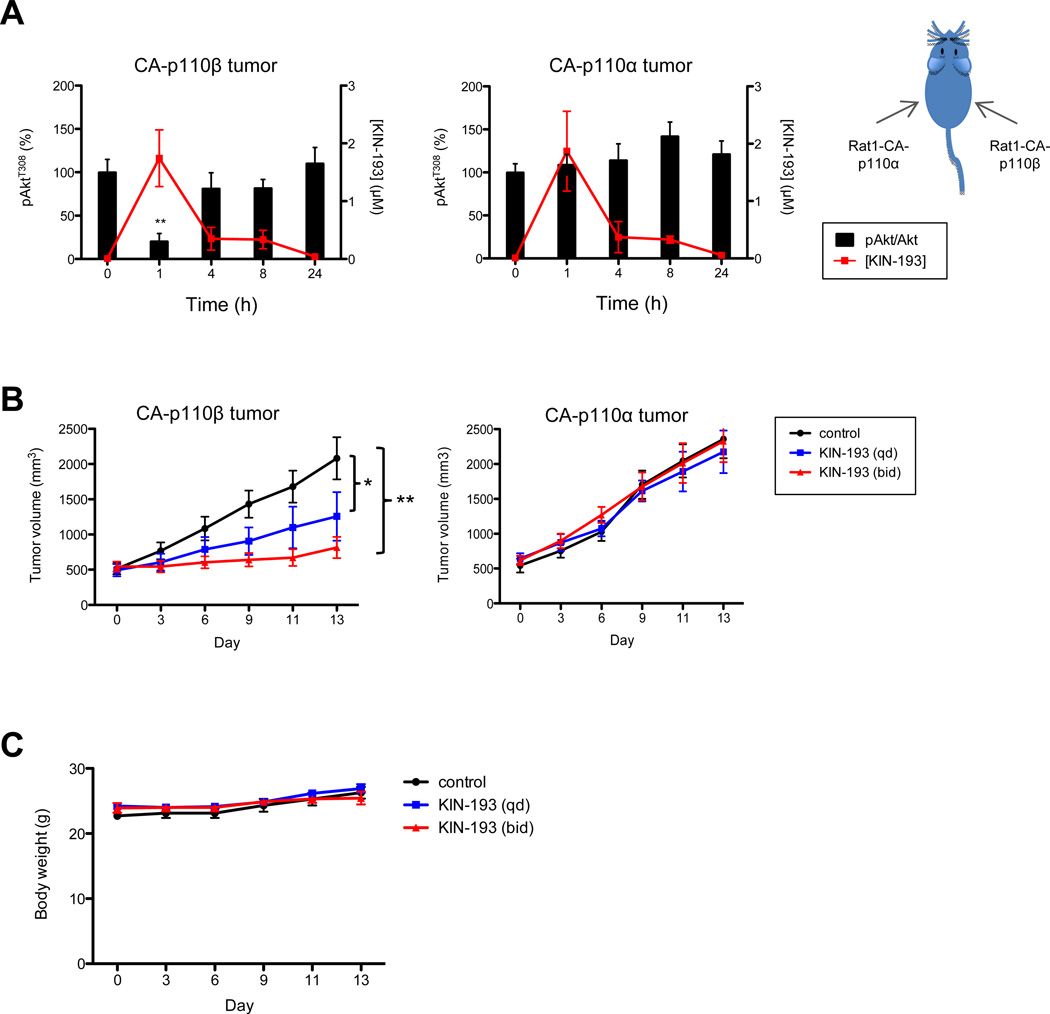
| In Vivo | To determine the pharmacodynamics of AZD 6482 (KIN-193) in tumors in vivo, rat fibroblast (Rat1) cells are engineered to express both p53DD, a dominant negative mutant of p53, and a constitutively activated myr-p110β (Rat1-CA-p110β) to enable these cells to form xenograft tumors in mice. For comparison, an isogenic Rat1 cell line expressing p53DD and myr-p110α (Rat1-CA-p110α) is also generated. Rat1-CA-p110α and Rat1-CA-p110β cells are introduced subcutaneously into the contralateral flanks of athymic mice such that tumors driven by activated p110α or p110β would be exposed to identical conditions and that concern about animal-to-animal variability could be eliminated. When tumors reach a volume of ~500 mm3, the tumor-bearing mice receives a single IP injection of AZD 6482 (10 mg/kg). The plasma concentration of AZD 6482 is highest at 1 hour post-injection and declined to undetectable levels by 4h. Concentrations of AZD 6482 in both the CA-p110α- and CA-p110β-driven tumors parallel the plasma concentrations. Analyses of tumor lysates harvested at various time points after AZD 6482 injection reveal that the phosphorylation of AKT is significantly reduced at 1hour after AZD 6482 injection in Rat1-CA-p110β tumors, but remain unchanged in Rat1-CAp110α tumors. | |
| Animal model | Mice; Approximately 6-8 week-old female nude mice are injected s.c. with Rat1-Myr-HA-p110α(Rat1-CAp110α) cells (1×106 cells in 40% matrigel) in one flank (site 1) and Rat1-Myr-HA-p110β (Rat1-CAp110β) cells (0.5×106 cells in 10% matrigel) in the contralateral flank (site 2). When tumors grow to ~500 mm3, mice are dosed once by ip injection with AZD 6482 formulated in 7.5% NMP, 40% PEG400, 52.5% dH2O at 0.1 mL/10g body weight and 10 mg/kg. Tumors are collected at 0, 1, 4, 8, and 24 h following compound administration and blood samples are obtained by direct heart puncture. Serum is separated and stored at -80°C. The drug concentrations in serum and tumor samples are assessed by LC-MS/MS analysis by the DMPK group. |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.4483 mL | 12.2414 mL | 24.4828 mL | 48.9656 mL |
| 5mM | 0.4897 mL | 2.4483 mL | 4.8966 mL | 9.7931 mL |
| 10mM | 0.2448 mL | 1.2241 mL | 2.4483 mL | 4.8966 mL |
| 20mM | 0.1224 mL | 0.6121 mL | 1.2241 mL | 2.4483 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.