This product is for research use only, not for human use. We do not sell to patients.
| Size | Price |
|---|---|
| 250mg | Get quote |
| 500mg | Get quote |
| 1g | Get quote |
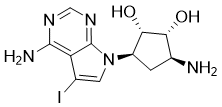
Cat #: V2024 CAS #: 186141-75-3 Purity ≥ 98%
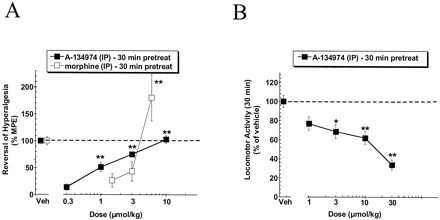
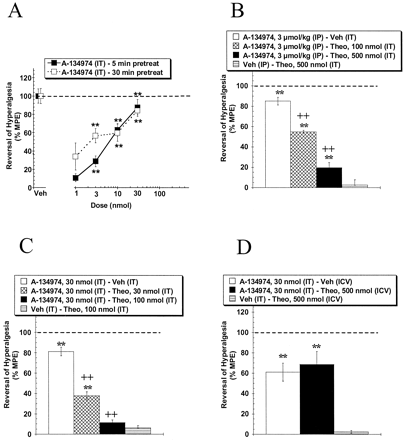
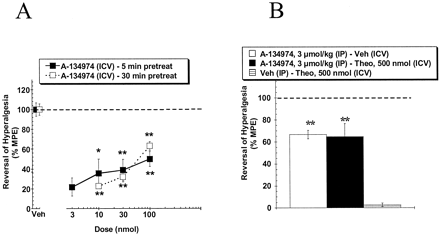
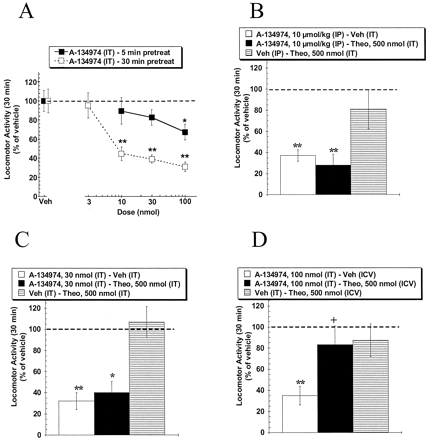
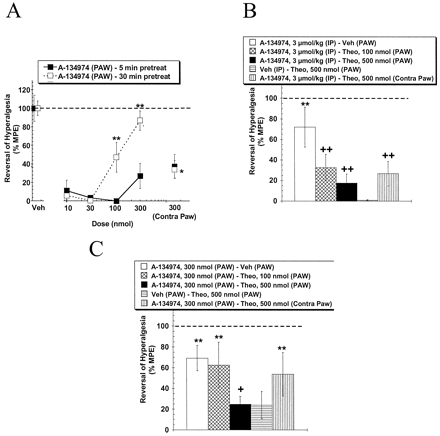
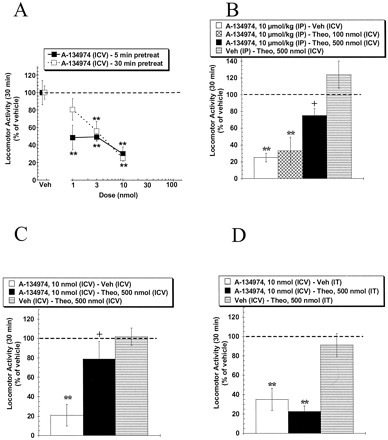
Description: A134974 is a novel, potent and selective adenosine kinase (AK) inhibitor with IC50 of 60 pM. Systemic A-134974 (i.p.) dose dependently reduced hyperalgesia (ED(50) = 1 micromol/kg) and at higher doses, reduced locomotor activity (ED(50) = 16 micromol/kg). Administration of A-134974 intrathecally (i.t.) was more potent (ED(50) = 6 nmol) at producing antihyperalgesia than delivering the compound by intracerebralventricular (ED(50) = 100 nmol, i.c.v.) or intraplantar (ED(50) >300 nmol) routes. In contrast, i.c.v. administration of A-134974 was more effective in reducing locomotor activity than i.t. administration (ED(50) values were 1 and >100 nmol, respectively).
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 375.17 |
|---|---|
| Molecular Formula | C11H14IN5O2 |
| CAS No. | 186141-75-3 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 10 mM |
| Water: N/A | |
| Ethanol: N/A | |
| Synonyms | A134974; A 134974; A-134974 |
| Protocol | In Vitro | In vitro activity: AK enzyme inhibition was assayed radiochemically as described byYamada et al. (1980) and McNally et al. (1997). The ability of A-134974 to inhibit AK activity in intact IMR-32 neuroblastoma cells (American Type Culture Collection, Gaithersburg, MD) carried out as previously described (Kowaluk and Cowart, 1994). Radioligand binding assay methodology for the A1, A2A, and A3 receptors was carried out as described by Jarvis et al. (2000). The ability of A-134974 to inhibit [3H]nitrobenzylthioinosine binding to the ADO transporter and to inhibit adenosine deaminase activity was also examined using previously described methodology (Parkinson and Geiger, 1996). |
|---|---|---|
| In Vivo | Drugs administered to rats were A-134974 (Cowart, 1997, an AK inhibitor synthesized at Abbott Laboratories), morphine sulfate (Mallinckrodt, St. Louis, MO), and theophylline (a nonselective ADO receptor antagonist; Sigma Chemical Co., St. Louis, MO). All drugs were dissolved in sterile water for local (intraplantar, i.t., or i.c.v.) or systemic (i.p.) delivery. The drug administration procedures described below were followed for locomotor activity experiments as well as for hyperalgesia experiments. Each experimental group consisted of at least five animals. | |
| Animal model | Male Sprague-Dawley rats (260–320 g) |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.6655 mL | 13.3273 mL | 26.6546 mL | 53.3092 mL |
| 5mM | 0.5331 mL | 2.6655 mL | 5.3309 mL | 10.6618 mL |
| 10mM | 0.2665 mL | 1.3327 mL | 2.6655 mL | 5.3309 mL |
| 20mM | 0.1333 mL | 0.6664 mL | 1.3327 mL | 2.6655 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.