This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 2mg | $180 | 3-6 Days |
| 5mg | $280 | 3-6 Days |
| 10mg | $440 | 3-6 Days |
| 25mg | $750 | 3-6 Days |
| 50mg | $1250 | 3-6 Days |
| 100mg | $1750 | 3-6 Days |
| 250mg | $2850 | 3-6 Days |
Cat #: V2736 CAS #: 1616420-30-4 Purity ≥ 98%
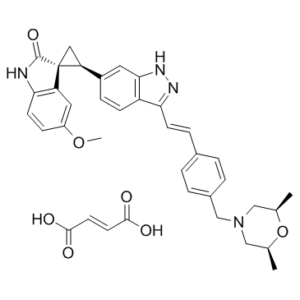
Description: CFI400945 (CFI-400945) fumarate is a potent, selective, and orally bioavailable PLK4 (polo-like kinase 4) inhibitor with IC50 value of 2.8 ±1.4 nM and potential antineoplastic activity. CFI-400945 selectively inhibits PLK4, which results in the disruption of mitosis and the induction of apoptosis. PLK4 inhibition also prevents cell division and inhibits proliferation of PLK4-overexpressing tumor cells. CFI-400945 can attenuate the growth of breast cell line, as well as other tumor cell lines significantly. CFI-400945 selectively inhibits PLK4 in cells, but also has certain activity against AURKB, TRKA, TRKB and Tie2/TEK (only 10 kinases showed more than 50% inhibition among 290 kinases).
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 650.72 |
|---|---|
| Molecular Formula | C37H38N4O7 |
| CAS No. | 1616420-30-4 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 100 mg/mL (187.0 mM) |
| Water:<1 mg/mL | |
| Ethanol: 100 mg/mL (187.0 mM) | |
| Synonyms | CFI400945 fumarate; CFI 400945 fumarate; CFI-400945 fumarate. |
| Protocol | In Vitro | In vitro activity: CFI-400945 is a potent, orally active antitumor agent with the IC50 and Ki values of 2.8 and 0.26 nM, respectively. It has no significant inhibition against PLKs 1-3 is observed for CFI-400945 at a concentration of 50 μM. CFI-400945 can attenuate the growth of breast cell line, as well as other tumor cell lines significantly. CFI-400945 selectively inhibits PLK4 in cells, but also has certain activity against AURKB, TRKA, TRKB and Tie2/TEK (only 10 kinases showed more than 50% inhibition among 290 kinases). The cytokinesis failure and subsequent polyploidization by CFI-400945 treatment indicate that the cell death in cancer cell lines is at least partly achieved through inhibition of AURKB. No significant inhibition is observed for PLKs 1-3 (IC50s > 50 μM) probably due to the most divergent structure of PLK4 compared to other polo-like kinases 1-3. Cancer cells treated with CFI-400945 exhibit effects consistent with PLK4 kinase inhibition, including dysregulated centriole duplication, mitotic defects, and cell death. Kinase Assay: Active PLK4 is purified and used to measure PLK4 activity, using an indirect ELISA detection system. PLK1, PLK2, PLK3, AURKA, and AUKB/INCENP compound inhibition are determined using FRET-based homogeneous assay kits from Invitrogen. The assays are performed with ATP concentrations of 25, 60, and 80 μM, respectively, for PLK1, PLK2, and PLK3 and ATP concentrations of 20 and 128 μM, respectively, for AURKA and AURKB/INCENP. Cell Assay: MDA-MB-468, MCF-7, HCC1954, MDA-MB-231, SKBr-3, Cal-51, and BT-20 breast cancer cells are seeded into 96-well plates at 3000, 4000, 4000, 2500, 4000, 3000, and 6000 cells per 80 μL, respectively, 24 h before compound overlay and cultured at 37℃ and 5% CO2. Compounds are prepared as 10 mM stocks in 100% DMSO. Each 10 mM stock is diluted with DMEM (Dulbecco's Modified Eagle's Medium) cell growth medium containing 10% FBS such that the final concentrations ranged from 50 nM to 250 μM. Aliquots (20 μL) from each concentration are overlaid to 80 μL of preseeded cells to achieve final concentrations of 10 nM to 50 μM. After 5 d, the cells are fixed in situ by gently removing the culture media and adding 50 μL of ice-cold 10% trichloroacetic acid (TCA) per well and incubation at 4 °C for 30 min. The plates are washed with water five times and allowed to air-dry for 5 min. Then 50 μL of 0.4% (w/v) sulforhodamine B (SRB) solution in 1% (v/v) acetic acid is added to each well, followed by incubation for 30 min at rt. The plates are washed four times with 1% acetic acid to remove unbound SRB and air-dried for 5 min. The SRB is solubilized with 100 μL of 10 mM Tris pH 10.5 per well, and absorbance is read at 570 nm. The percentage (%) of relative inhibition of cell viability is calculated. |
|---|---|---|
| In Vivo | CFI-400945 is well tolerated in breast cancer xenograft models, in particular those deficient in the tumor suppressor PTEN. Upon intermittent oral dosing, in a mouse model of colon cancer, CFI-400945 is an effective inhibitor of HCT116 tumor growth and was well tolerated. CFI-400945 is absorbed rapidly after oral administration, reaching maximum plasma concentrations (Cmax) of 0.25-11.68 μg/mL for the doses tested (3.75-104 mg/kg). CFI-400945 can inhibit the growth of a range of tumor types and may be effective in a clinical setting even in advanced tumors. Following oral administration of efficacious doses of CFI-400945 in mice, plasma levels of CFI-400945 are sustained and remained above both the EC50 value for half-maximal inhibition of cellular PLK4 autophosphorylation and growth inhibition GI50 values for 24 hr. Moreover, CFI-400945 demonstrates dose-dependent antitumor activity. Analysis of xenograft tumors from mice treated with an efficacious dose of CFI-400945 shows a pharmacodynamic effect that is suggestive of complete rather than partial inhibition of PLK4 kinase activity. | |
| Animal model | Adult female athymic CD1 nude mice |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 1.5368 mL | 7.6838 mL | 15.3676 mL | 30.7352 mL |
| 5mM | 0.3074 mL | 1.5368 mL | 3.0735 mL | 6.1470 mL |
| 10mM | 0.1537 mL | 0.7684 mL | 1.5368 mL | 3.0735 mL |
| 20mM | 0.0768 mL | 0.3842 mL | 0.7684 mL | 1.5368 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































