This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 500mg | $50 | 3-6 Days |
| 1g | $68 | 3-6 Days |
| 2g | $88 | 3-6 Days |
| 5g | $115 | 3-6 Days |
| 10g | $160 | 3-6 Days |
| 100g | $800 | 3-6 Days |
Cat #: V1764 CAS #: 13311-84-7 Purity ≥ 98%
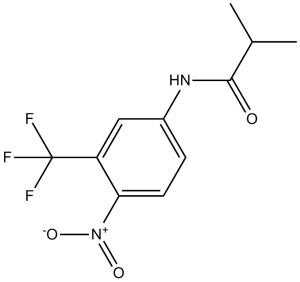
Description: Flutamide (also known as SCH13521; SCH-13521; trade names: Flucinom; Flugerel; Niftolid; Flutan; Oncosal etc.), a toluidine derivative, is a potent non-steroidal antiandrogen drug (AR antagonist) with potential anticancer activity. It is mainly used for the treatment of prostate cancer. Flutamide can be transformed into an active metablolite that binds to androgen receptor with a Ki value of 55 nM. Flutamideis a toluidine derivative and nonsteroidal antiandrogen that is structurally related to bicalutamide and nilutamide. Flutamide and the more active metabolite 2-hydroxyflutamide competitively block dihydrotestosterone binding at androgen receptors, forming inactive complexes which cannot translocate into the cell nucleus.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 276.21 |
|---|---|
| Molecular Formula | C11H11F3N2O3 |
| CAS No. | 13311-84-7 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 55 mg/mL (199.1 mM) |
| Water: <1 mg/mL | |
| Ethanol: 55 mg/mL (199.1 mM) | |
| Synonyms | SCH-13521; SCH13521; SCH 13521; trade names: Flucinom; Flugerel; Niftolid; Flutan; Oncosal; Profamid; Prostacur; Flutaplex; Fugerel; Grisetin; Eulexin; Apimid; Chimax; Drogenil; Euflex; Eulexine; Flucinome; Fluken; Flulem; Flutabene; Flutacan; FlutaGry; Flutamex; Flutamin; Prostadirex; Prostica; Prostogenat; Tafenil; Tecnoflut; Testotard. FLUT. |
| Protocol | In Vitro | In vitro activity: Flutamide (Eulexin) is an antiandrogen drug. Flutamide-OH, the active metabolite of flutamide, directly binds at rat anterior pituitary androgen receptor with Ki values of 55 nM. Flutamide does not affect the proliferation of an androgen-sensitive clone of the mouse mammary carcinoma Shionogi SC-l 15 cells in culture, shows only antiandrogenic effect, but not androgenic effect. Flutamide provides treatment for prostate cancer when used along with leuprolide. Kinase Assay: Aliquots of 100 μl cytosol are incubated at 0-4°C for 18 h with 100 μl of the indicated saturating concentration of [3H]T in the presence or absence of increasing concentrations of nonlabeled T, DHT, flutamide (FLU) or flutamide-OH (FLU-OH). At the end of the incubation, free and bound T are separated by the addition of 200 μl dextran-coated charcoal (1 % charcoal, 0.1% dextran T-70, 0.1% gelatin, 1.5 mM EDTA and 50 mM Tris (pH 7.4)) for 15 min before centrifugation at 2300 × g for another 15 min at 0-4°C. Aliquots (350 μl) of the supernatant are transferred to scintillation vials with 10 ml of an aqueous counting solution before counting in a Beckman LS 330 counter. Cell Assay: Effect of flutamide on the growth of an androgen-sensitive clone (SEM-l) of mouse mammary carcinoma Shionogi cells in culture. The cells are incubated up to 40 days in medium (MEM + 2% dextran-coated charcoal extracted fetal calf serum) containing the compounds at a concentration of 1 μM. Media are changed every second day. |
|---|---|---|
| In Vivo | Flutamide causes a markedly reduction in rat ventral prostate weight from 319 mg to 245 mg. A combination of flutamide and LHRH agonist induces an additive effect with a decrease in prostate weight to 101 mg, and an marked drop in prostatic ODC activity. | |
| Animal model | Male rat |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 3.6204 mL | 18.1022 mL | 36.2043 mL | 72.4087 mL |
| 5mM | 0.7241 mL | 3.6204 mL | 7.2409 mL | 14.4817 mL |
| 10mM | 0.3620 mL | 1.8102 mL | 3.6204 mL | 7.2409 mL |
| 20mM | 0.1810 mL | 0.9051 mL | 1.8102 mL | 3.6204 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































