This product is for research use only, not for human use. We do not sell to patients.
| Size | Price | Stock |
|---|---|---|
| 500mg | $800 | Check With Us |
| 1g | $1250 | Check With Us |
| 5g | $3150 | Check With Us |
Cat #: V0647 CAS #: 244240-24-2 Purity ≥ 98%
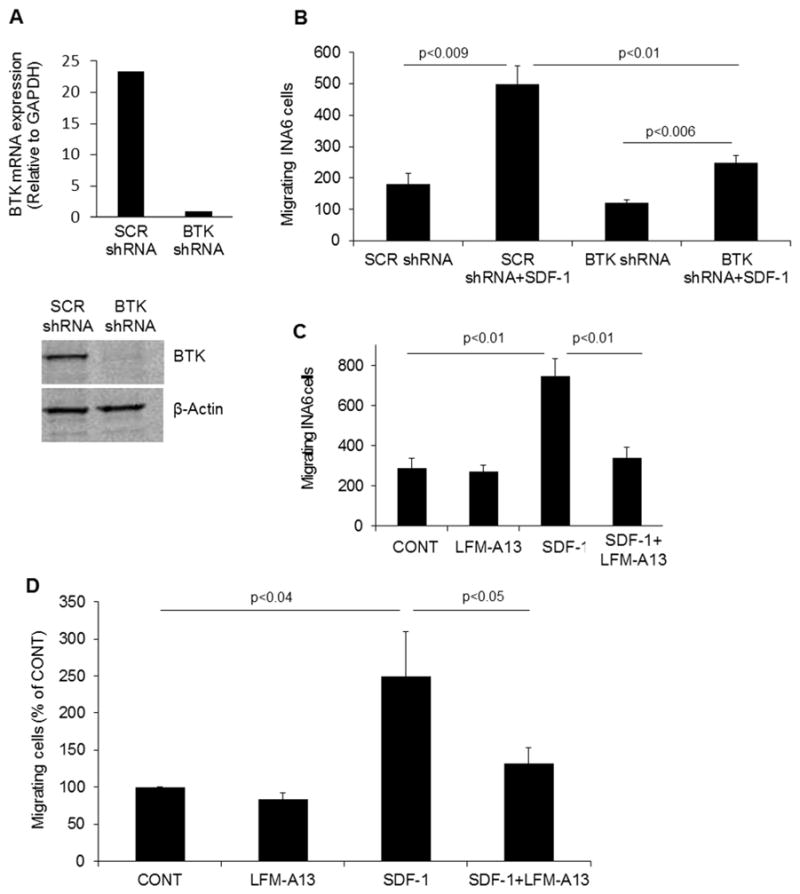
Description: LFM-A13 (LFM-A1-3) is a novel, potent and specific Bruton's tyrosine kinase (BTK) inhibitor with potential anticancer activity. It inhibits BTK with an IC50 of 2.5 μM, and shows >100-fold selectivity over other protein kinases such as JAK1, JAK2, HCK, EGFR,and IRK. LFM-A13 inhibited recombinant BTK expressed in a baculovirus expression vector system. Besides its remarkable potency in BTK kinase assays, LFM-A13 was also found to be a highly specific inhibitor of Polo-like kinases. LFM-A13 shows high in vivo anticancer efficacy in BALB/c mice bearing BCL-1 leukemia.
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Weight (MW) | 360 |
|---|---|
| Molecular Formula | C11H8Br2N2O2 |
| CAS No. | 244240-24-2 |
| Storage | -20℃ for 3 years in powder formr |
| -80℃ for 2 years in solvent | |
| Solubility In Vitro | DMSO: 72 mg/mL (200.0 mM)r |
| Water: <1 mg/mLr | |
| Ethanol: <1 mg/mL | |
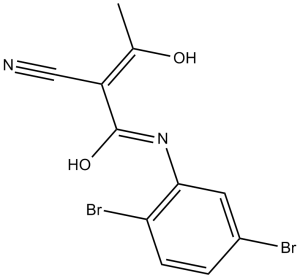
| SMILES Code | C/C(O)=C(C#N)/C(NC1=CC(Br)=CC=C1Br)=O |
| Synonyms | LFM-A13; LFM A13; LFM A13 |
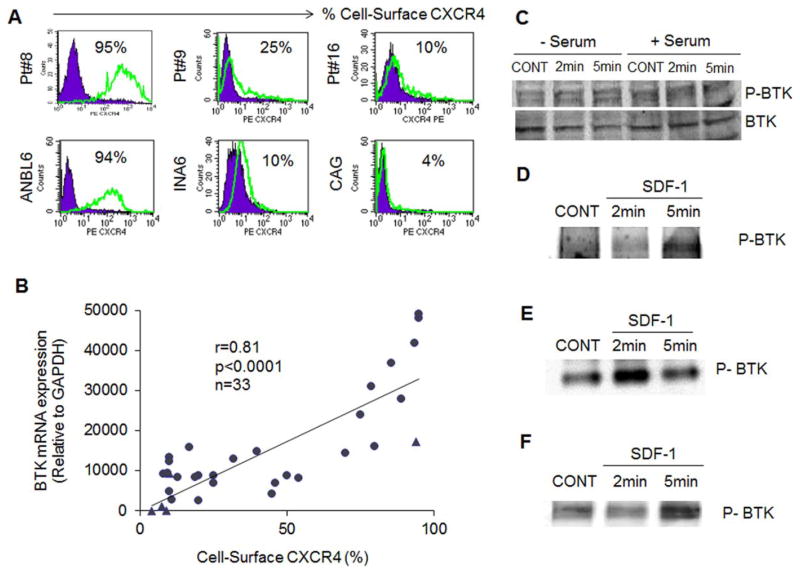
| Protocol | In Vitro | In vitro activity: In BTK+ B-lineage leukemic cells, LFM-A13 enhances their sensitivity to ceramide- or vincristine-induced apoptosis. In BCL-1 cells, NALM-6 cells, or normal BALB/c splenocytes, LFM-13 inhibits the enzymatic activity of BTK in BCL-1 cells without affecting the BTK protein expression levels. In human neutrophils, LFM-A13 decreases the tyrosine phosphorylation induced by fMet-Leu-Phe and inhibits the production of superoxide anions and the stimulation of adhesion, chemotaxis, and phospholipase D activity. Kinase Assay: Purified His6-Plx1 (250 ng) is added to a 20 μL reaction mixture containing 1× kinase buffer (10 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and 1 mM TT), 25 μM cold ATP, and 1 μCi [γ-32P]ATP in the presence of different concentrations of LFM-A13 ranging from 5 μg/mL (13.9 μM) to 100 μg/mL (278 μM). The reaction mixtures are incubated at room temperature for 15-30 min and autophosphorylation is stopped by addition of 2× SDS-PAGE reducing sample buffer. A parallel experiment is performed in the presence of cold ATP. The kinase reactions are then subjected to immunoblotting using the commercially available anti-Plk antibodies. The immunoblots confirmed that the same amount of Plx1 protein is present in each reaction. In addition, we also examined the effects of LFM-A13 on substrate phosphorylation by Plx1. In brief, 250 ng of purified Plx1 is first incubated at room temperature for one hour with different concentrations of LFM-A13. After one hour of incubation, the tubes containing the reaction mixtures are put on ice and the substrate, GST-Cdc25 peptide (254-316) (200 ng), kinase buffer, and [γ-32P]ATP are added and the kinase reaction allowed to proceed for 15 min at room temperature. Immunoblotting with anti-Cdc25 antibodies is used to confirm that equal amounts of the substrate peptide are present in each reaction mixture. Anti-Plk antibodies, the polyclonal antibodies to gluthathione-S-transferase (GST) and ECL kit are used in the assay. The mode of human PLK3 inhibition by LFM-A13 is examined in titration experiments using increasing concentrations of [γ-32P]ATP and purified N-terminal His6-tagged recombinant human PLK3, residues 19-301, expressed by baculovirus in Sf21 insect cells. In brief, in a final reaction volume of 25 μL, PLK3 (h) (5-10 mU) is incubated with 8 mM MOPS, pH 7.0, 0.2 mM EDTA, 2 mg/mL casein, 10 mM Mg acetate, and [γ-32P-ATP] (specific activity approx. 500 cpm/pmol, concentration as required). The reaction is initiated by the addition of the MgATP mix. After incubation for 40 min at room temperature, the reaction is stopped by the addition of 5 μL of a 3% phosphoric acid solution. Ten microliters of the reaction is then spotted onto a P30 filtermat and washed three times for 5 min in 75 mM phosphoric acid and once in methanol prior to drying and scintillation counting. The Ki of PLK3 by LFM-A13 is calculated from the reciprocal plots of the intensity of phosphorylation of the substrate (1/v) versus the concentration of the inhibitor (i) (viz., LFM-A13). From this Dixon plot, the Ki represents the dissociation constants of the EI complex, which is determined by the point of linear intersection. Cell Assay: LFM-A13 (100 μM) suppresses Epo-induced phosphorylation of EpoR, Jak2, Btk, Stat5 and Erk1/2 in R10 cells. LFM-A13 (100 μM) inhibits auto-phosphorylation of Jak2, Tec and Btk rather than Lyn kinase auto-phosphorylation in COS cells. LFM-A13 potently inhibits Plx1 with IC50 of 10 μM; also inhibits BRK, BMX, FYN and with IC50s of 267, 281, 240 and 215 μM. |
|---|---|---|
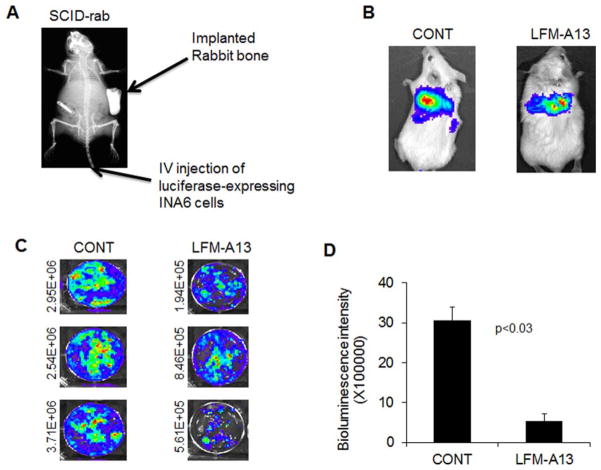
| In Vivo | In BALB/c mice bearing BCL-1 leukemia, combination of LFM-A13 (50 mg/kg/day i.p.) and the standard triple-drug VPL prolongs the median survival time. In primary myeloma-bearing SCID-rab mice, LFM-A13 inhibits osteoclast activity, prevents myeloma-induced bone resorption and suppresss myeloma growth. | |
| Animal model | BALB/c mice bearing BCL-1 leukemia |
| Solvent volume to be added | Mass (the weight of a compound) | |||
|---|---|---|---|---|
| Mother liquor concentration | 1mg | 5mg | 10mg | 20mg |
| 1mM | 2.7778 mL | 13.8889 mL | 27.7778 mL | 55.5556 mL |
| 5mM | 0.5556 mL | 2.7778 mL | 5.5556 mL | 11.1111 mL |
| 10mM | 0.2778 mL | 1.3889 mL | 2.7778 mL | 5.5556 mL |
| 20mM | 0.1389 mL | 0.6944 mL | 1.3889 mL | 2.7778 mL |
This equation is commonly abbreviated as: C1 V1 = C2 V2
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.