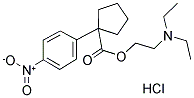
Nitrocaramiphen hydrochloride
This product is for research use only, not for human use. We do not sell to patients.
For small sizes, please check our retail website as below: www.invivochem.com
| Size | Price | Stock |
|---|---|---|
| 250mg | $1700 | Check With Us |
| 500mg | $2500 | Check With Us |
| 1μg | $2750 | Check With Us |
Cat #: V4121 CAS #: 98636-73-8 Purity ≥ 98%
Description: Nitrocaramiphen hydrochloride is a M1 muscarinic (mAChR) antagonist which demonstrates 71-fold selectivity for M 1 over M 2.
References: Eur J Pharmacol. 1993 Feb 16;231(3):485-8.
Top Publications Citing Invivochem Products
Publications Citing InvivoChem Products
Product Promise

- Physicochemical and Storage Information
- Protocol
- Related Biological Data
- Stock Solution Preparation
- Quality Control Documentation
| Molecular Formula | C18H26N2O4 HCl |
|---|---|
| CAS No. | 98636-73-8 |
| Protocol | In Vitro | Caramiphen, iodocaramiphen and nitrocaramiphen were examined for affinity at the muscarinic M1, M2 and M3 receptor subtypes in radioligand binding assays. Caramiphen binds with high affinity at the M1 site labeled by [3H]pirenzepine in rat cortex (Ki = 1.2 nM) and displays a 27-fold greater preference for the M1 than the M2 site labeled by [3H](-)-quinuclidinyl benzilate in rat heart, and a 6-fold greater preference for the M1 than the M3 site labeled by [3H]N-methylscopolamine in rat submaxillary gland. Iodocaramiphen binds with high affinity (Ki = 2.1 nM) and selectivity (59-fold) for the M1 vs. M2 subtype, and is 4-fold more selective for the M1 vs. M3 site. Nitrocaramiphen binds with high affinity for M1 sites (Ki = 5.5 nM) and with a 71-fold selectivity over M2, and a 10-fold selectivity for the M1 over the M3 subtype. All three compounds interacted with the M1 binding site in a competitive manner. Nitrocaramiphen and iodocaramiphen are as potent and showed a comparable selectivity for binding to the M1 over the M2 site than the prototypical agent pirenzepine (M1; Ki = 5.2 nM, 51-fold selectivity). Additionally, nitrocaramiphen demonstrates at least a 10-fold selectivity for the M1 over the M3 site. These ester-type antimuscarinics may be better ligands for the study of M1 receptors in brain than the hydrophilic agent pirenzepine. |
|---|
These protocols are for reference only. InvivoChem does not
independently validate these methods.
The molarity calculator equation
Mass(g) = Concentration(mol/L) × Volume(L) × Molecular Weight(g/mol)
Mass
=
Concentration
×
Volume
×
Molecular Weight*
The dilution calculator equation
Concentration(start)
×
Volume(start)
=
Concentration(final)
×
Volume(final)
This equation is commonly abbreviated as: C1 V1 = C2 V2
Concentration(start)
C1
×
Volume(start)
V1
=
Concentration(final)
C2
×
Volume(final)
V2
Step One: Enter information below
Dosage mg/kg
Average weight of animals g
Dosing volume per animal µL
Number of animals
Step Two: Enter the in vivo formulation
%DMSO
+
%
+
%Tween 80
+
%ddH2O
Calculation Results:
Working concentration:
mg/ml;
Method for preparing DMSO master liquid:
mg
drug pre-dissolved in
µL
DMSO(Master liquid concentration
mg/mL)
,Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation:
Take
µL
DMSO master liquid, next add
µL
PEG300, mix and clarify, next add
µL
Tween 80,mix and clarify, next add
µL
ddH2O,mix and clarify.
Note:
- (1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
- (2) Be sure to add the solvent(s) in order.




































